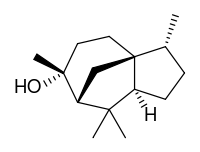
Cedrol
 | |
| Names | |
|---|---|
| IUPAC name
(1S,2R,5S,7R,8R)-2,6,6,8-tetramethyltricyclo[5.3.1.01,5]undecan-8-ol | |
| Identifiers | |
| 77-53-2 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1974890 |
| ChemSpider | 59018 |
| ECHA InfoCard | 100.000.942 |
| PubChem | 65575 |
| |
| |
| Properties | |
| C15H26O | |
| Molar mass | 222.37 g·mol−1 |
| Density | 1.01 g/mL |
| Melting point | 86 to 87 °C (187 to 189 °F; 359 to 360 K)[1] |
| Boiling point | 273 °C (523 °F; 546 K)[2] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cedrol is a sesquiterpene alcohol found in the essential oil of conifers (cedar oil), especially in the genera Cupressus (cypress) and Juniperus (juniper). It has also been identified in Origanum onites, a plant related to oregano.[3] Its main uses are in the chemistry of aroma compounds.[4] It makes up about 19% of cedarwood oil Texas and 15.8% of cedarwood oil Virginia.[5]
Cedrol has toxic and possibly carcinogenic properties.[6] Results of a 2015 study suggest that cedrol strongly attracts pregnant female mosquitoes after they have fed, which can be used to create cedrol-baited traps.[7]
See also
- Cedrene, another component of cedar oil
References
- ↑ Budavari, Susan, ed. (1996), The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (12th ed.), Merck, ISBN 0911910123, 1961
- ↑ Sigma-Aldrich Co., (+)-Cedrol. Retrieved on 25 May 2011.
- ↑ Connolly, JD; Hill, RA, eds. (1991). Dictionary of Terpenoids. Voume 1 Mono- and sesquiterpenoids. Chapman&Hall. SQ02555. ISBN 0-412-25770-X.
- ↑ Breitmeier, E (2006). Terpenes: flavors, fragrances, pharmaca, pheromones. Wiley-VCH. pp. 46–47. ISBN 3-527-31786-4.
- ↑ Susan Barclay-Nichols. "Point of Interest!". swiftcraftymonkey.blogspot.com.
- ↑ Sabine, J.R. (1975). "Exposure to an environment containing the aromatic red cedar, Juniperus virginiana: procarcinogenic, enzyme-inducing and insecticidal effects.". Toxicology. 5 (2): 221–235. doi:10.1016/0300-483x(75)90119-5. PMID 174251.
- ↑ Lindh, Jenny; Okal, Michael (March 2015). "Discovery of an oviposition attractant for gravid malaria vectors of the Anopheles gambiae species complex". Malaria Journal. 14 (119). doi:10.1186/s12936-015-0636-0.
This article is issued from Wikipedia - version of the 5/28/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.