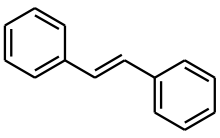
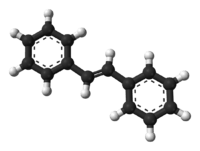
(E)-Stilbene
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,1'-[(1E)-Ethene-1,2-diyl]dibenzene | |
| Other names
(E)-1,2-Diphenylethene (E)-Stilbene trans-Stilbene trans-1,2-Diphenylethylene | |
| Identifiers | |
| 103-30-0 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:36007 |
| ChEMBL | ChEMBL113028 |
| ChemSpider | 553649 |
| ECHA InfoCard | 100.002.817 |
| PubChem | 638088 |
| UNII | 3FA7NW80A0 |
| |
| |
| Properties | |
| C14H12 | |
| Molar mass | 180.25 g·mol−1 |
| Appearance | Solid |
| Density | 0.9707 g/cm3 |
| Melting point | 122 to 125 °C (252 to 257 °F; 395 to 398 K) |
| Boiling point | 305 to 307 °C (581 to 585 °F; 578 to 580 K) |
| Practically insoluble | |
| Hazards | |
| Safety data sheet | External MSDS |
| NFPA 704 | |
| Flash point | >112 °C (234 °F) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
(E)-Stilbene, is the organic compound with the formula (C6H5CH)2. Classified as a diarylethene, it features a central ethene double bond substituted with phenyl groups on each carbon atoms of the double bond. The name stilbene is derived from the Greek word stilbos, which means shining. It is a white solid that dissolves in organic solvents.
Isomers

Stilbene exists as two possible isomers. One is trans-1,2-diphenylethylene, called (E)-stilbene or trans-stilbene. The second is cis-1,2-diphenylethylene, called (Z)-stilbene or cis-stilbene, and is sterically hindered and less stable because the steric interactions force the aromatic rings out-of-plane and prevent conjugation. (Z)-Stilbene has a melting point of 5–6 °C (41–43 °F), while (E)-stilbene melts around 125 °C (257 °F), illustrating the two compounds are quite different in their physical properties.[1]
Preparation and reactions
Many syntheses have been developed. One popular route entails reduction of benzoin using zinc.[2]
Stilbene undergoes reactions typical of alkenes, being susceptible to bromination, epoxidation, and cycloaddition. Upon irradiation it converts to cis-stilbene, a classic example of a photochemical reaction involving trans-cis isomerization.
Derivatives and uses
Stilbene itself is of little value but serves as a precursor to other derivatives used as dyes, optical brighteners, phosphors, and scintillators. Stilbene is one of the gain mediums used in dye lasers.
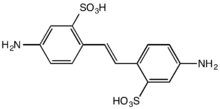
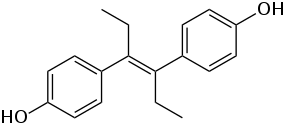
The stilbenoids are naturally occurring stilbene derivatives. Examples include resveratrol and its cousin, pterostilbene. The stilbestrols, which are structurally but not synthetically related to E-stilbene, exhibit estrogenic activity. Members of this group include diethylstilbestrol, fosfestrol, and dienestrol.
References
- ↑ Robert E. Buckles and Norris G. Wheeler "cis-Stilbene" Org. Synth. 1953, volume 33, p. 88. doi:10.15227/orgsyn.033.0088
- ↑ R. L. Shriner and Alfred Berger "trans-Stilbene" Org. Synth. 1943, volume 23, 86. doi:10.15227/orgsyn.023.0086
Appendix
Table 1. Vapor pressures[1]
| Isomer | Temperature, °C | Vapor pressure, kPa |
|---|---|---|
| cis-stilbene | 100 | 0.199 |
| cis-stilbene | 125 | 0.765 |
| cis-stilbene | 150 | 2.51 |
| trans-stilbene | 150 | 0.784 |
External links
- ↑ Lide, David (1995). CRC Handbook of Chemistry and Physics (76th ed.). USA: CRC Press, Inc. pp. 6–107. ISBN 0-8493-0476-8.
