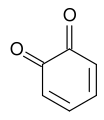
1,2-Benzoquinone
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Cyclohexa-3,5-diene-1,2-dione | |||
| Other names | |||
| Identifiers | |||
| 583-63-1 | |||
| 3D model (Jmol) | Interactive image Interactive image | ||
| ChEBI | CHEBI:17253 | ||
| ChemSpider | 10941 | ||
| KEGG | C02351 | ||
| PubChem | 11421 | ||
| UNII | SVD1LJ47R7 | ||
| |||
| |||
| Properties | |||
| C6H4O2 | |||
| Molar mass | 108.0964 g/mol | ||
| Density | 1.424 g/cm3 | ||
| Boiling point | 213.3 °C (415.9 °F; 486.4 K) at 760 mmHg | ||
| Hazards | |||
| Flash point | 76.4 °C (169.5 °F; 349.5 K) | ||
| Related compounds | |||
| Related compounds |
1,4-benzoquinone quinone | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
1,2-Benzoquinone, also called ortho-benzoquinone, is an organic compound with formula | C=6 | H=4 | O=2 . It is one of the two isomers of quinone, the other being 1,4-benzoquinone. It is a red volatile solid that is soluble in water and ethyl ether. It is rarely encountered because of its instability, but it is of fundamental interest as the parent ortho-quinone, of which many analogues are known.[2]
Structure
The molecule has C2v symmetry. X-ray crystallography shows that the double bonds are localized, with alternatingly long and short C-C distances within the ring. The C=O distances of 1.21 Å are characteristic of ketones.[3]
Preparation and occurrence
1,2-Benzoquinone is produced on oxidation of catechol exposed to air in aqueous solution[4] or by ortho oxidation of a phenol.[4] It is a precursor to melanin.[5]
A strain of the bacterium Pseudomonas mendocina metabolises benzoic acid, yielding 1,2-benzoquinone via catechol.[6]
References
- 1 2 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 728. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Liao, Chun-Chen; Peddinti, Rama Krishna "Masked o-Benzoquinones in Organic Synthesis" Accounts of Chemical Research 2002, volume 35, pp. 856-866.doi:10.1021/ar000194n
- ↑ Macdonald, Alistair L.; Trotter, James "Crystal and molecular structure of o-benzoquinone" Journal of the Chemical Society, Perkin Transactions 2: 1973, pp. 476-80. doi:10.1039/P29730000476
- 1 2 Magdziak, D.; Rodriguez, A. A.; Van De Water, R. W.; Pettus, T. R. R. (2002). "Regioselective oxidation of phenols to o-quinones with o-iodoxybenzoic acid (IBX)". Org. Lett. 4 (2): 285–288. doi:10.1021/ol017068j. PMC 1557836
 . PMID 11796071.
. PMID 11796071. - ↑ Enzymatic Browning in Fruits, Vegetables and Seafoods Section 2.3.2
- ↑ Chanda Parulekar and Suneela Mavinkurve (2006), Formation of ortho-benzoquinone from sodium benzoate by Pseudomonas mendocina | P=2 d. Indian Journal of Experimental Biology, volume 44, pages 157--162.