Dantron
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, rectal (enema) |
| ATC code | A06AB03 (WHO) A06AG03 (WHO) |
| Identifiers | |
| |
| CAS Number |
117-10-2 |
| PubChem (CID) | 2950 |
| DrugBank |
DB04816 |
| ChemSpider |
2845 |
| UNII |
Z4XE6IBF3V |
| KEGG |
D07107 |
| ChEBI |
CHEBI:3682 |
| ChEMBL |
CHEMBL53418 |
| NIAID ChemDB | 001375 |
| ECHA InfoCard | 100.003.794 |
| Chemical and physical data | |
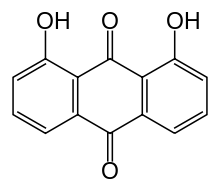
| Formula | C14H8O4 |
| Molar mass | 240.211 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Dantron (INN), also known as chrysazin or 1,8-dihydroxyanthraquinone, is an organic substance, formally derived from anthraquinone by the replacement of two hydrogen atoms by hydroxyl groups (–OH). It is used in some countries as a stimulant laxative.
It should not be confused with ondansetron, an unrelated drug that was marketed in South Africa under the trade name "Dantron".
Medical uses
In the USA, dantron is not used because it is considered to be a carcinogen.[1]
In the UK it is considered a possible carcinogen and so its licence is restricted to patients who already have a diagnosis of terminal cancer. It is mainly used in palliative care to counteract the constipating effects of opioids. Its British Approved Name was danthron, but it has now been changed to "dantron", the recommended International Nonproprietary Name.[2]
Dantron has the notable side-effect of causing red-colored urine.
See also
References
- ↑ Danthron substance profile at the National Toxicology Program website
- ↑ British National Formulary website (requires free registration)