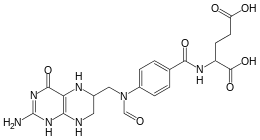
10-Formyltetrahydrofolate
 | |
 | |
| Names | |
|---|---|
| IUPAC names
(2S)-2-{[4-[(2-amino-4-oxo-5,6,7,8-tetrahydro-1H-pteridin-6-yl) methyl(formyl)amino]benzoyl]amino}pentanedioic acid | |
| Other names
10-CHO-THF | |
| Identifiers | |
| 2800-34-2 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 9 |
| MeSH | 10-formyl-tetrahydrofolate |
| PubChem | 10 |
| |
| |
| Properties | |
| C20H23N7O7 | |
| Molar mass | 473.44 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
10-Formyltetrahydrofolate (10-CHO-THF) is a form of tetrahydrofolate that acts as a donor of formyl groups in anabolism. In these reactions 10-CHO-THF is used as a substrate in formyltransferase reactions. This is important in purine biosynthesis, where 10-CHO-THF is a substrate for phosphoribosylaminoimidazolecarboxamide formyltransferase, as well as in the formylation of the methionyl initiator tRNA (fMet-tRNA), when 10-CHO-THF is a substrate for methionyl-tRNA formyltransferase.[1]
Biosynthesis
10-CHO-THF is produced either by the enzyme methenyltetrahydrofolate cyclohydrolase via the reaction
- 5,10-methenyltetrahydrofolate + H2O 10-formyltetrahydrofolate
or by the enzyme formate-tetrahydrofolate ligase via the reaction
- ATP + formate + tetrahydrofolate ADP + phosphate + 10-formyltetrahydrofolate
It can be converted back into tetrahydrofolate (THF) by formyltetrahydrofolate dehydrogenase or THF and formate by formyltetrahydrofolate deformylase.