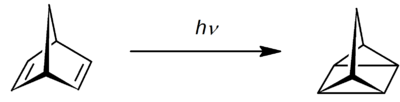Norbornadiene
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Bicyclo[2.2.1]hepta-2,5-diene | |||
| Other names
2,5-Norbornadiene | |||
| Identifiers | |||
| 121-46-0 | |||
| 3D model (Jmol) | Interactive image | ||
| ECHA InfoCard | 100.004.066 | ||
| EC Number | 204-472-0 | ||
| PubChem | 8473 | ||
| |||
| Properties | |||
| C7H8 | |||
| Molar mass | 92.14 g/mol | ||
| Density | 0.906 g/cm3 | ||
| Melting point | −19 °C (−2 °F; 254 K) | ||
| Boiling point | 89 °C (192 °F; 362 K) | ||
| Insoluble | |||
| Hazards | |||
| R-phrases | R11 | ||
| S-phrases | S9 S16 S29 S33 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Norbornadiene is a bicyclic, hydrocarbon and an organic compound. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerize (isomers would be anti-Bredt olefins). Norbornadiene is also a useful dienophile in Diels-Alder reactions.
Synthesis
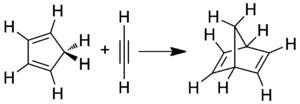
Norbornadiene can be formed by a Diels-Alder reaction between cyclopentadiene and acetylene.
Reactions
Quadricyclane, a valence isomer, can be obtained from norbornadiene by a photochemical reaction when assisted by a sensitizer such as acetophenone:[1]
The norbornadiene-quadricyclane couple is of potential interest for solar energy storage when controlled release of the strain energy stored in quadricyclane back to norbornadiene is made possible.[2]
Norbornadiene is reactive in cycloaddition reactions. Norbornadiene is also the starting material for the synthesis of diamantane[3] and sumanene and it is used as an acetylene transfer agent for instance in reaction with 3,6-di-2-pyridyl-1,2,4,5-tetrazine.[4]
As a ligand
Norbornadiene is a versatile ligand in organometallic chemistry, where it serves as a two-electron or four-electron donor. The norbornadiene analogue of cyclooctadiene rhodium chloride dimer is widely used in homogeneous catalysis. Chiral, C2-symmetric dienes derived from norbornadiene have also been described.[5]
One example is tetracarbonyl(norbornadiene)chromium(0),[6] which is a useful source of "chromium tetracarbonyl," e.g. in reactions with phosphine ligands.
References
- ↑ Smith, Claiborune D. (1988). "Quadricyclane". Org. Synth.; Coll. Vol., 6, p. 962
- ↑ Gregory W. Sluggett; Nicholas J. Turro & Heinz D. Roth (1997). "Rh(III)-Photosensitized Interconversion of Norbornadiene and Quadricyclane". J. Phys. Chem. A. 101 (47): 8834–8838. doi:10.1021/jp972007h.
- ↑ Diamantane in Organic Syntheses Coll. Vol. 6, p.378; Vol. 53, p.30 Online Article
- ↑ Ronald N. Warrener & Peter A. Harrison (2001). "π-Bond Screening in Benzonorbornadienes: The Role of 7-Substituents in Governing the Facial Selectivity for the Diels-Alder Reaction of Benzonorbornadienes with 3,6-Di(2-pyridyl)-s-Tetrazine" (PDF). Molecules. 6 (4): 353–369. doi:10.3390/60400353.
- ↑ Ryo Shintani, Tamio Hayashi "Chiral Diene Ligands for Asymmetric Catalysis" Aldrich Chimica Acta 2009, vol. 42, number 2, pp. 31-38.
- ↑ Markus Strotmann; Rudolf Wartchow & Holger Butenschön (2004). "High yield synthesis and structures of some achiral and chiral (diphosphine)tetracarbonylchromium(0) chelate complexes with tetracarbonyl(norbornadiene)chromium(0) as complexation reagent". Arkivoc: KK–1112F.