Activity-dependent plasticity
Activity-dependent plasticity is a form of functional and structural neuroplasticity that arises from the use of cognitive functions and personal experience;[1] hence, it is the biological basis for learning and the formation of new memories.[1][2] Activity-dependent plasticity is a form of neuroplasticity that arises from intrinsic or endogenous activity, as opposed to forms of neuroplasticity that arise from extrinsic or exogenous factors, such as electrical brain stimulation- or drug-induced neuroplasticity.[1] The brain's ability to remodel itself forms the basis of the brain’s capacity to retain memories, improve motor function, and enhance comprehension and speech amongst other things. It is this trait to retain and form memories that is associated with neural plasticity and therefore many of the functions individuals perform on a daily basis.[3] This plasticity occurs as a result of changes in gene expression which are triggered by signaling cascades that are activated by various signaling molecules (e.g., calcium, dopamine, and glutamate) during increased neuronal activity.[4]
The brain’s ability to adapt toward active functions allows humans to specialize in specific processes based on relative use and activity. For example, a right-handed person may perform any movement poorly with his/her left hand but continuous practice with the less dominant hand can make both hands just as able. Another example is if someone was born with a neurological disorder such as autism or had a stroke that resulted in a disorder, then they are capable of retrieving much of their lost function by practicing and “rewiring” the brain in order to incorporate these lost manners.[5] Thanks to the pioneers within this field, many of these advances have become available to most people and many more will continue to arrive as new features of plasticity are discovered.
History
During the first half of the 1900s, the word ‘plasticity’ was considered foul and directly and indirectly rejected throughout science. Many scientists found it hard to receive funding because nearly everyone unanimously supported the fact that the brain was fully developed at adulthood and specific regions were unable to change functions after the critical period. It was believed that each region of the brain had a set and specific function. Despite closed-mindedness and ignorance, several pioneers pushed the idea of plasticity through means of various experiments and research. There are others that helped to the current progress of activity-dependent plasticity but the following contributed very effective results and ideas early on.
Pioneers of activity-dependent plasticity
The history of activity-dependent plasticity begins with Paul Bach y Rita. With conventional ideology being that the brain development is finalized upon adulthood, Bach y Rita designed several experiments in the late 1960s and 1970s that proved that the brain is capable of changing. These included a pivotal visual substitution method for blind people provided by tactile image projection in 1969.[6] The basis behind this experiment was to take one sense and use it to detect another: in this case use the sense of touch on the tongue to visualize the surrounding. This experiment was years ahead of its time and lead to many questions and applications. A similar experiment was reported again by Bach y Rita in 1986 where vibrotactile stimulation was delivered to the index fingertips of naive blindfolded subjects.[7] Even though the experiment did not yield great results, it supported the study and proposed further investigations. In 1998, his design was even further developed and tested again with a 49-point electrotactile stimulus array on the tongue.[8] He found that five sighted adult subjects recognized shapes across all sizes 79.8% of the time, a remarkable finding that has led to the incorporation of the tongue electrotactile stimulus into cosmetically acceptable and practical designs for blind people. In later years, he has published a number of other articles including "Seeing with the brain" in 2003 where Bach y Rita addresses the plasticity of the brain relative to visual learning.[9] Here, images are enhanced and perceived by other plastic mechanisms within the realm of information passing to the brain.
Another pioneer within the field of activity-dependent plasticity is Michael Merzenich, currently a professor in neuroscience at the University of California, San Francisco. One of his contributions includes mapping out and documenting the reorganization of cortical regions after alterations due to plasticity.[10] While assessing the recorded changes in the primary somatosensory cortex of adult monkeys, he looked at several features of the data including how altered schedules of activity from the skin remap to cortical modeling and other factors that affect the representational remodeling of the brain. His findings within these studies have since been applied to youth development and children with language-based learning impairments. Through many studies involving adaptive training exercises on computer, he has successfully designed methods to improve their temporal processing skills. These adaptive measures include word-processing games and comprehension tests that involve multiple regions of the brain in order to answer. The results later translated into his development of the Fast Forword program in 1996, which aims to enhance cognitive skills of children between kindergarten and twelfth grade by focusing on developing “phonological awareness.”[11] It has proven very successful at helping children with a variety of cognitive complications. In addition, it has led to in depth studies of specific complications such as autism and intellectual disability and the causes of them.[12] Alongside a team of scientists, Merzenich helped to provide evidence that autism probes monochannel perception where a stronger stimulus-driven representation dominates behavior and weaker stimuli are practically ignored in comparison.
Structure of neurons
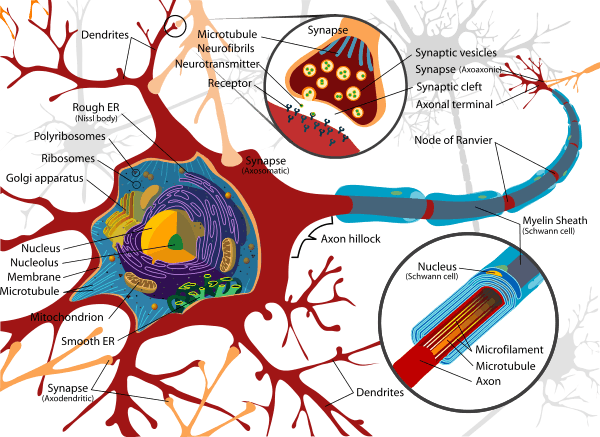
Neurons are the basic functional unit of the brain and process and transmit information through signals. Many different types of neurons can be identified based on their function, such as sensory neurons or motor neurons. Each responds to specific stimuli and sends respective and appropriate chemical signals to other neurons. The basic structure of a neuron is shown here on the right and consists of a nucleus that contains genetic information; the cell body, or the soma, which is equipped with dendritic branches that mostly receive the incoming inputs from other neurons; a long, thin axon that bears axon terminals which carry the output information to other neurons.[13] The dendrites and axons are interfaced through a small connection called a synapse. This component of the neuron contains a variety of chemical messengers and proteins that allow for the transmission of information. It is the variety of proteins and affect of the signal that fundamentally lead to the plasticity feature.
Structures and molecular pathways involved
Activity-dependent plasticity of one form or another has been observed in most areas of the brain. In particular, it is thought that the reorganization of sensory and motor maps involves a variety of pathways and cellular structures related to relative activity.
Many molecules have been implicated in synaptic plasticity. Notably, AMPA and NMDA receptors are key molecules in mechanisms of long and short-term potentiation between neurons. NMDA receptors can detect local activity due to activation and therefore modify signaling in the post-synaptic cell. The increased activity and coordination between pre- and post-synaptic receptors leads to more permanent changes and therefore result in plasticity. Hebb’s postulate addresses this fact by stating that synaptic terminals are strengthened by correlated activity and will therefore sprout new branches. However, terminals that experience weakened and minimal activity will eventually lose their synaptic connection and deteriorate.[14]
A major target of all molecular signaling is the inhibitory connections made by GABAergic neurons. These receptors exist at postsynaptic sites and along with the regulation of local inhibitory synapses have been found to be very sensitive to critical period alterations. Any alteration to the receptors leads to changed concentrations of calcium in the affected cells and can ultimately influence dendritic and axonal branching.[15] This concentration change is the result of many kinases being activated, the byproduct of which may enhance specific gene expression.

In addition, it has been identified that the wg postsynaptic pathway, which is responsible for the coding and production of many molecules for development events, can be bidirectionally stimulated and is responsible for the downstream alteration of the postsynaptic neuron. When the wg presynaptic pathway is activated, however, it alters cytoskeletal structure through transcription and translation.[16]
Cell adhesion molecules (CAMs) are also important in plasticity as they help coordinate the signaling across the synapse. More specifically, integrins, which are receptors for extracellular matrix proteins and involved with CAMs, are explicitly incorporated in synapse maturation and memory formation. They play a crucial role in the feedback regulation of excitatory synaptic strength, or long-term potentiation (LTP), and help to control synaptic strength by regulating AMPA receptors, which result in quick, short synaptic currents.[17] But, it is the metabotropic glutamate receptor 1 (mGlu1) that has been discovered to be required for activity-dependent synaptic plasticity in associative learning.[18]
Activity-dependent plasticity is seen in the primary visual cortex, a region of the brain that processes visual stimuli and is capable of modifying the experienced stimuli based on active sensing and arousal states. It is known that synaptic communication trends between excited and depressed states relative to the light/dark cycle. By experimentation on rats, it was found that visual experience during vigilant states leads to increased responsiveness and plastic changes in the visual cortex.[19] More so, depressed states were found to negatively alter the stimulus so the reaction was not as energetic. This experiment proves that even the visual cortex is capable of achieving activity-dependent plasticity as it is reliant on both visual exploration and the arousal state of the animal.
Role in learning
Activity-dependent plasticity plays a very important role in learning and in the ability of understanding new things. It is responsible for helping to adapt an individual’s brain according to the relative amount of usage and functioning. In essence, it is the brain’s ability to retain and develop memories based on activity-driven changes of synaptic strength that allow stronger learning of information. It is thought to be the growing and adapting quality of dendritic spines that provide the basis for synaptic plasticity connected to learning and memory.[20] Dendritic spines accomplish this by transforming synaptic input into neuronal output and also by helping to define the relationship between synapses.
In recent studies, a specific gene has also been identified as having a strong role in synapse growth and activity-dependent plasticity: the microRNA 132 gene (miR132).[21] This gene is regulated by the cAMP response element-binding (CREB) protein pathway and is capable of enhancing dendritic growth when activated. The miR132 gene is another component that is responsible for the brain’s plasticity and helps to establish stronger connections between neurons.
Another plasticity-related gene involved in learning and memory is Arc/Arg3.1. The Arc gene is activity-regulated[22] and the transcribed mRNA is localized to activated synaptic sites[23][24] where the translated protein plays a role in AMPA receptor trafficking.[25] Arc is a member of a class of proteins called immediate early genes (IEG) that are rapidly transcribed in response to synaptic input. Of the estimated 30-40 genes that comprise the total neuronal IEG response, all are prototypical activity-dependent genes and a number have been implicated in learning and memory. For example, zif268, Arc, beta-activin, tPA, Homer, and COX-2 have all been implicated in long-term potentiation (LTP),[26] a cellular correlate of learning and memory.
Mechanisms involved
There are a variety of mechanisms involved in activity-dependent plasticity. These include LTP, long-term depression (LTD), synaptic elimination, neurogenesis, and synaptogenesis.[3] The mechanisms of activity-dependent plasticity result in membrane depolarization and calcium influx, which in turn trigger cellular changes that affect synaptic connections and gene transcription. In essence, neuronal activity regulates gene expression related to dendritic branching and synapse development. Mutations in activity-dependent transcription-related genes can lead to neurological disorders. Each of the studies’ findings aims to help proper development of the brain while improving a wide variety of tasks such as speech, movement, comprehension, and memory. More so, the findings better explain the development induced by plasticity.
It is known that during postnatal life a critical step to nervous system development is synapse elimination. The changes in synaptic connections and strength are results from LTP and LTD and are strongly regulated by the release of brain-derived neurotrophic factor (BDNF), an activity-dependent synapse-development protein.[27][28] In addition to BDNF, Nogo-66 receptors, and more specifically NgR1, are also involved in the development and regulation of neuronal structure.[29] Damage to this receptor leads to pointless LTP and attenuation of LTD. Both situations imply that NgR1 is a regulator of synaptic plasticity. From experiments, it has been found that stimulation inducing LTD leads to a reduction in synaptic strength and loss of connections but, when coupled simultaneously with low-frequency stimulation, helps the restructuring of synaptic contacts. The implications of this finding include helping people with receptor damage and providing insight into the mechanism behind LTP.
Another research model of activity-dependent plasticity includes the excitatory corticostriatal pathway that is involved in information processing related to adaptive motor behaviors and displays long-lasting synaptic changes. The change in synaptic strength is responsible for motor learning and is dependent on the simultaneous activation of glutamatergic corticostriatal and dopaminergic nigrostriatal pathways. These are the same pathways affected in Parkinson's disease, and the degeneration of synapses within this disorder may be responsible for the loss of some cognitive abilities.[30] Therefore, the impairment of DA/ACh-dependent learning can lead to the storage of inessential memories .
Relationship to behavior
Intellectual disability
Since plasticity is such a fundamental property of brain function due to its involvement in brain development, brain repair, and cognitive processes, its proper regulation is necessary for normal physiology. Mutations within any of the genes associated with activity-dependent plasticity have been found to positively correlate with various degrees of intellectual disability.[31] The two types of intellectual disability related to plasticity depend on dysfunctional neuronal development or alterations in molecular mechanisms involved in synaptic organization. Complications within either of these types can greatly reduce brain capability and comprehension.
Stroke rehabilitation
On the other hand, people with such conditions have the capacity to recover some degree of their lost abilities through continued challenges and use. A great example of this can be seen within Norman Doidge’s ‘The Brain That Changes Itself.’ Bach y Rita’s father suffered from a disabling stroke that left the 65-year-old man half-paralyzed and unable to speak. After one year of crawling and unusual therapy tactics including playing basic children’s games and washing pots, his father’s rehabilitation was nearly complete and he went back to his role as a professor at City College in New York.[32] The remarkable recovery from a stroke proves that even someone with abnormal behavior and severe medical complications can recover nearly all of the normal functions by much practice and perseverance: thus the message behind activity-dependent plasticity.
Recent studies have reported that a specific gene, FMR1, is highly involved in activity-dependent plasticity and Fragile X syndrome (FraX) is the result of this gene’s loss of function. FMR1 produces FMRP, which mediates activity-dependent control of synaptic structure. The loss or absence of this gene almost certainly leads to both autism and intellectual disability. Dr. Gatto has found that early introduction of the product FMRP results in nearly complete restructuring of the synapses. This method is not as effective, though, when introduced into a mature subject and only partially accommodates for the losses of FMR1.[33] The discovery of this gene provides a possible location for intervention for young children with these abnormalities as this gene and its product act early to construct synaptic architecture.
Stress
A common issue amongst most people in the United States is high levels of stress and also disorders associated with continuous stress. Many regions of the brain are very sensitive to stress and can be damaged with extended exposure. More importantly, many of the mechanisms involved with increased memory retention, comprehension, and adaptation are thought to involve LTP and LTD, two activity-dependent plasticity mechanisms that stress can directly suppress. Several experiments have been conducted in order to discover the specific mechanisms for this suppression and also possible intervention methods. Dr. Li and several others have actually identified the TRPV1 channel as a target to facilitate LTP and suppress LTD, therefore helping to protect the feature of synaptic plasticity and retention of memory from the effects of stress.[34]
Future studies
The future studies and questions for activity-dependent plasticity are nearly endless because the implications of the findings will enable many treatments. Despite many gains within the field, there are a wide variety of disorders that further understanding of activity-dependent mechanisms of plasticity would help treat and perhaps cure. These include autism, different severities of intellectual disability, schizophrenia, Parkinson’s Disease, stress, and stroke. In addition to a better understanding of the various disorders, neurologists should and will look at the plasticity incurred by the immune system, as it will provide great insight into diseases and also give the basis of new immune-centered therapeutics.[35] A better perspective of the cellular mechanisms that regulate neuronal morphology is the next step to discovering new treatments for learning and memory pathological conditions.
See also
References
- 1 2 3 Ganguly K, Poo MM (October 2013). "Activity-dependent neural plasticity from bench to bedside". Neuron. 80 (3): 729–741. doi:10.1016/j.neuron.2013.10.028. PMID 24183023.
Much progress has been made in understanding how behavioral experience and neural activity can modify the structure and function of neural circuits during development and in the adult brain. Studies of physiological and molecular mechanisms underlying activity-dependent plasticity in animal models have suggested potential therapeutic approaches for a wide range of brain disorders in humans. Physiological and electrical stimulations as well as plasticity-modifying molecular agents may facilitate functional recovery by selectively enhancing existing neural circuits or promoting the formation of new functional circuits. ... Neural plasticity can be broadly defined as the ability of the nervous system to adopt a new functional or structural state in response to extrinsic and intrinsic factors. Such plasticity is essential for the development of the nervous system and normal functioning of the adult brain. Neural plasticity can manifest at the macroscale as changes in the spatiotemporal pattern of activation of different brain regions, at the mesoscale as alterations of long-range and local connections among distinct neuronal types, and at the microscale as modifications of neurons and synapses at the cellular and subcellular levels. Maladaptive neural plasticity may account for many developmental, acquired, and neurodegenerative brain disorders.
- ↑ Keller TA, Just MA (January 2016). "Structural and functional neuroplasticity in human learning of spatial routes". Neuroimage. 125: 256–266. doi:10.1016/j.neuroimage.2015.10.015. PMID 26477660.
Recent findings with both animals and humans suggest that decreases in microscopic movements of water in the hippocampus reflect short-term neuroplasticity resulting from learning. Here we examine whether such neuroplastic structural changes concurrently alter the functional connectivity between hippocampus and other regions involved in learning. ... These concurrent changes characterize the multidimensionality of neuroplasticity as it enables human spatial learning.
- 1 2 Bruel-Jungerman E, Davis S, Laroche S (October 2007). "Brain plasticity mechanisms and memory: a party of four". Neuroscientist. 13 (5): 492–505. doi:10.1177/1073858407302725. PMID 17901258.
A defining characteristic of the brain is its remarkable capacity to undergo activity-dependent functional and morphological remodeling via mechanisms of plasticity that form the basis of our capacity to encode and retain memories. Today, it is generally accepted that the neurobiological substrate of memories resides in activity-driven modifications of synaptic strength and structural remodeling of neural networks activated during learning.
- ↑ Flavell SW, Greenberg ME (2008). "Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system". Annu. Rev. Neurosci. 31: 563–90. doi:10.1146/annurev.neuro.31.060407.125631. PMC 2728073
 . PMID 18558867.
. PMID 18558867. Sensory experience and the resulting synaptic activity within the brain are critical for the proper development of neural circuits. Experience-driven synaptic activity causes membrane depolarization and calcium influx into select neurons within a neural circuit, which in turn trigger a wide variety of cellular changes that alter the synaptic connectivity within the neural circuit. One way in which calcium influx leads to the remodeling of synapses made by neurons is through the activation of new gene transcription. Recent studies have identified many of the signaling pathways that link neuronal activity to transcription, revealing both the transcription factors that mediate this process and the neuronal activity–regulated genes. These studies indicate that neuronal activity regulates a complex program of gene expression involved in many aspects of neuronal development, including dendritic branching, synapse maturation, and synapse elimination.
Figure 3: Calcium-induced signal transduction networks mediating neuronal activity-dependent gene expression. - ↑ Doidge, Norman (2007). The Brain That Changes Itself: Stories of personal triumph from the frontiers of brain science. New York: Penguin Group. ISBN 978-0-14-311310-2.
- ↑ Bach-y-Rita P, Collins CC, Sauders F, White B, Scadden L (1969). "Vision substitution by tactile image projection". Nature. 221 (5184): 963–64. doi:10.1038/221963a0. PMID 5818337.
- ↑ Epstein W, Hughes B, Schneider S, Bach-y-Rita P (1986). "Is anything out there? A study of distal attribution in response to vibrotactile stimulation". Perception. 15 (3): 275–84. doi:10.1068/p150275. PMID 3797201.
- ↑ Bach-y-Rita P, Kaczmarek K, Tyler M, Garcia-Lara J (1998). "Form perception with a 49-point electrotactile stimulus array on the tongue". Rehab Research Devel. 35: 427–30.
- ↑ Bach-y-Rita P, Tyler ME, Kaczmarek KA (2003). "Seeing with the brain". International Journal of Human-Computer Interaction. 15 (2): 285–95. doi:10.1207/s15327590ijhc1502_6.
- ↑ Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM (2001). "Sensory input directs spatial and temporal plasticity in primary auditory cortex". J. Neurophys. 86 (1): 326–38.
- ↑ Fast ForWord Website
- ↑ Bonneh YS; et al. (2008). "Cross-modal extinction in a boy with severely autistic behaviour and high verbal intelligence". Cogn Neuropsychol. 25 (5): 635–52. doi:10.1080/02643290802106415. PMID 18651259.
- ↑ Purves, Dale; George J. Augustine; David Fitzpatrick; William C. Hall; Anthony-Samuel LaMantia; James O. McNamara; Leonard E. White (2008). Neuroscience, 4th Ed. Sunderland, MA: Sinauer Associates, Inc. pp. 3–11. ISBN 978-0-87893-697-7.
- ↑ Purves, Dale; George J. Augustine; David Fitzpatrick; William C. Hall; Anthony-Samuel LaMantia; James O. McNamara; Leonard E. White (2008). Neuroscience, 4th Ed. Sunderland, MA: Sinauer Associates, Inc. pp. 625–26. ISBN 978-0-87893-697-7.
- ↑ Purves, Dale; George J. Augustine; David Fitzpatrick; William C. Hall; Anthony-Samuel LaMantia; James O. McNamara; Leonard E.r White (2008). Neuroscience, 4th Ed. Sunderland, MA: Sinauer Associates, Inc. pp. 630–32. ISBN 978-0-87893-697-7.
- ↑ Ataman B; et al. (2008). "Rapid Activity-Dependent Modifications in Synaptic Structure and Function Require Bidirectional Wnt Signaling". Neuron. 57 (5): 705–18. doi:10.1016/j.neuron.2008.01.026. PMC 2435264
 . PMID 18341991.
. PMID 18341991. - ↑ Cingolani LA; et al. (2007). "Activity-Dependent Regulation of Synaptic AMPA Receptor Composition and Abundance by β3 Integrins". Neuron. 58 (5): 749–62. doi:10.1016/j.neuron.2008.04.011. PMC 2446609
 . PMID 18549786.
. PMID 18549786. - ↑ Gil-Sanz C, Delgado-Garcia JM, Fairen A, Gruart A (2008). "Involvement of the mGluR1 receptor in hippocampal synaptic plasticity and associative learning in behaving mice". Cerebral Cortex. 18 (7): 1653–63. doi:10.1093/cercor/bhm193. PMID 18024992.
- ↑ Tsanov M, Manahan-Vaughn D (2007). "Intrinsic, light-independent and visual activity-dependent mechanisms cooperate in the shaping of the field response in rat visual cortex". J. Neurosci. 27 (31): 8422–29. doi:10.1523/jneurosci.1180-07.2007. PMID 17670989.
- ↑ Sala C, Cambianica I, Rossi F (2008). "Molecular mechanisms of dendritic spine development and maintenance". Acta Neurobiol. Exp. 68 (2): 289–304.
- ↑ Wayman GA; et al. (2008). "An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP". Proceedings of the National Academy of Sciences of the United States of America. 105 (26): 9093–98. doi:10.1073/pnas.0803072105. PMC 2449370
 . PMID 18577589.
. PMID 18577589. - ↑ Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Worley PF (1995). "Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeletal-associated protein that is enriched in neuronal dendrites". Neuron. 14 (2): 433–445. doi:10.1016/0896-6273(95)90299-6. PMID 7857651.
- ↑ Wallace CS, Lyford GL, Worley PF, Steward O (1998). "Differential intracellular sorting of immediate early gene mRNAs depends on signals in the mRNA sequence". J Neurosci. 18 (1): 26–35. PMID 9412483.
- ↑ Steward O, Worley PF (2001). "Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation". Neuron. 30 (1): 227–240. doi:10.1016/S0896-6273(01)00275-6. PMID 11343657.
- ↑ Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF, et al. (2006). "Arc Interacts with the Endocytic Machinery to Regulate AMPA Receptor Trafficking". Neuron. 52 (3): 445–459. doi:10.1016/j.neuron.2006.08.033. PMC 1784006
 . PMID 17088211.
. PMID 17088211. - ↑ French PJ, O'Connor V, Jones MW, Davis S, Errington ML, Voss K, Truchet B, Wotjak C, Stean T, et al. (2001). "Subfield-specific immediate early gene expression associated with hippocampal long-term potentiation in vivo". Eur J Neurosci. 13 (5): 968–976. doi:10.1046/j.0953-816x.2001.01467.x. PMID 11264669.
- ↑ Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM (2008). "Synapse elimination accompanies functional plasticity in hippocampal neurons". Proc. Natl. Acad. Sci. USA. 105 (8): 3123–27. doi:10.1073/pnas.0800027105. PMC 2268595
 . PMID 18287055.
. PMID 18287055. - ↑ Jia J; et al. (2008). "Brain-derived neurotrophic factor-tropomyosin-related kinase B signaling contributes to activity-dependent changes in synaptic proteins". J. Biol. Chem. 283 (30): 21242–50. doi:10.1074/jbc.M800282200. PMC 3258936
 . PMID 18474605.
. PMID 18474605. - ↑ Lee HJ; et al. (2008). "Synaptic function for the Nogo-66 receptor NgR1: Regulation of dendritic spine morphology and activity-dependent synaptic strength". J. Neurosci. 28 (11): 2753–65. doi:10.1523/jneurosci.5586-07.2008. PMID 18337405.
- ↑ Calabresi P, Galletti F, Saggese E, Ghiglieri V, Picconi B (2007). "Neuronal networks and synaptic plasticity in Parkinson's disease: beyond motor deficits". Parkinsonism & Related Disorders. 13: S259–S262. doi:10.1016/S1353-8020(08)70013-0.
- ↑ Vaillend C, Poirier R, Laroche S (2008). "Genes, plasticity and mental retardation". Behav. Brain Res. 192 (1): 88–105. doi:10.1016/j.bbr.2008.01.009. PMID 18329113.
- ↑ Doidge, Norman (2007). The Brain That Changes Itself: Stories of personal triumph from the frontiers of brain science. New York: Penguin Group. pp. 20–24. ISBN 978-0-14-311310-2.
- ↑ Gatto CL, Broadie K (2008). "Temporal Requirements of the Fragile X Mental Retardation Protein in the Regulation of Synaptic Structure". Development. 135 (15): 2637–48. doi:10.1242/dev.022244. PMC 2753511
 . PMID 18579676.
. PMID 18579676. - ↑ Li HB, Mao RR, Zhang JC, Cao YJ, Xu L (2008). "Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory". Biological Psychiatry. 64 (4): 286–92. doi:10.1016/j.biopsych.2008.02.020. PMID 18405883.
- ↑ Di Filippo M, Sarchielli P, Picconi B, Calabresi P (2008). "Neuroinflammation and synaptic plasticity: theoretical basis for a novel, immune-centered, therapeutic approach to neurological disorders". Trends in Pharmacological Sciences. 29 (8): 402–12. doi:10.1016/j.tips.2008.06.005. PMID 18617277.