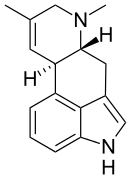
Agroclavine
 | |
| Names | |
|---|---|
| IUPAC name
(6aR,10aR)-7,9-Dimethyl-4,6,6a,7,8,10a-hexahydroindolo[4,3-fg]quinoline | |
| Other names
6,8-Dimethyl-8,9-didehydroergoline; 8,9-Didehydro-6,8-dimethylergoline | |
| Identifiers | |
| 548-42-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:2519 |
| ChEMBL | ChEMBL449081 |
| ChemSpider | 66176 |
| ECHA InfoCard | 100.008.135 |
| PubChem | 73484 |
| |
| |
| Properties | |
| C16H18N2 | |
| Molar mass | 238.33 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Agroclavine belongs to the group of ergot alkaloids, such as ergotamine.[1] Historically, the main use of argoclavine was to oxidize it to elymoclavine, which can then be used for ergot-based drug synthesis.
References
- ↑ Bhattacharji, S.; Birch, A. J.; Brack, A.; Hofmann, A.; Kobel, H.; Smith, D. C. C.; Smith, Herchel; Winter J. (1962). "Biosynthesis. XXVII. The biosynthesis of ergot alkaloids". Journal of the Chemical Society: 421–425.
This article is issued from Wikipedia - version of the 5/22/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.