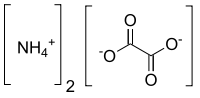
Ammonium oxalate
 | |
| Names | |
|---|---|
| IUPAC name
Diammonium ethanedioate | |
| Other names
Diammonium oxalate | |
| Identifiers | |
| 1113-38-8 6009-70-7 (monohydrate) | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:91241 |
| ChemSpider | 13577 |
| ECHA InfoCard | 100.012.912 |
| PubChem | 14213 |
| |
| |
| Properties | |
| C2H8N2O4 | |
| Molar mass | 124.10 g·mol−1 |
| Appearance | White solid |
| Melting point | 70 C (158 F, 343.15 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Ammonium oxalate, C2H8N2O4 - more commonly written as (NH4)2C2O4 - is an oxalate salt with ammonium (sometimes as a monohydrate). It is a colorless salt under standard conditions and is odorless and non-volatile. It is a strong dicarboxylic acid and occurs in many plants and vegetables. It is produced in the body by metabolism of glyoxylic acid or ascorbic acid. It is not metabolized but excreted in the urine.[1] It is a constituent of some types of kidney stone.[2][3] It is also found in guano.
Ammonium oxalate is used as an analytical reagent and general reducing agent.[1] It and other oxalates are used as anticoagulants, to preserve blood outside the body.
References
- 1 2 National Center for Biotechnology Information. PubChem Compound Database; CID 14213 (accessed 15 November 2016).
- ↑ The International Pharmacopoeia, p.1292, Volume 1, World Health Organization, 2006 ISBN 92-4-156301-X.
- ↑ N G Coley, "The collateral sciences in the work of Golding Bird (1814-1854)", Medical History, iss.4, vol.13, October 1969, pp.372.
This article is issued from Wikipedia - version of the 11/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.