Polyaniline
Polyaniline (PANI) is a conducting polymer of the semi-flexible rod polymer family. Although the compound itself was discovered over 150 years ago, only since the early 1980s has polyaniline captured the intense attention of the scientific community. This interest is due to the rediscovery of high electrical conductivity. Amongst the family of conducting polymers and organic semiconductors, polyaniline has many attractive processing properties. Because of its rich chemistry, polyaniline is one of the most studied conducting polymers of the past 50 years.[1]
History
As described by Alan MacDiarmid,[2] the first definitive report of polyaniline did not occur until 1862, which included an electrochemical method for the determination of small quantities of aniline.[3]
From the early 20th century on, occasional reports about the structure of PANI were published. Subsequent to his investigation of other highly-conductive organic materials, MacDiarmid demonstrated the conductive states of polyaniline which arose upon protonic doping of the emeraldine form of polyaniline.[4] Conductive polymers such as polyaniline remain of widespread interest,[5] providing an opportunity to address fundamental issues of importance to condensed matter physics, including, for example, the metal-insulator transition,[6] the Peierls Instability and quantum decoherence.[7]
Synthesis and properties
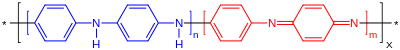
Polymerized from the inexpensive aniline monomer, polyaniline can be found in one of three idealized oxidation states:[8]
- leucoemeraldine – white/clear & colorless (C6H4NH)n
- emeraldine – green for the emeraldine salt, blue for the emeraldine base ([C6H4NH]2[C6H4N]2)n
- (per)nigraniline – blue/violet (C6H4N)n
In figure 1, x equals half the degree of polymerization (DP). Leucoemeraldine with n = 1, m = 0 is the fully reduced state. Pernigraniline is the fully oxidized state (n = 0, m = 1) with imine links instead of amine links. Studies have shown that most forms of polyaniline are one of the three states or physical mixtures of these components. The emeraldine (n = m = 0.5) form of polyaniline, often referred to as emeraldine base (EB), is neutral, if doped (protonated) it is called emeraldine salt (ES), with the imine nitrogens protonated by an acid. Protonation helps to delocalize the otherwise trapped diiminoquinone-diaminobenzene state. Emeraldine base is regarded as the most useful form of polyaniline due to its high stability at room temperature and the fact that, upon doping with acid, the resulting emeraldine salt form of polyaniline is highly electrically conducting.[2] Leucoemeraldine and pernigraniline are poor conductors, even when doped with an acid.
The colour change associated with polyaniline in different oxidation states can be used in sensors and electrochromic devices.[9] Although color is useful, the best method for making a polyaniline sensor is arguably to take advantage of the dramatic changes in electrical conductivity between the different oxidation states or doping levels.[10] Treatment of emeraldine with acids increases the electrical conductivity by ten orders of magnitude. Undoped polyaniline has a conductivity of 6.28×10−9 S/m, while conductivities of 4.60×10−5 S/m can be achieved by doping to 4% HBr.[11] The same material can be prepared by oxidation of leucoemeraldine.
Polyaniline is more noble than copper and slightly less noble than silver which is the basis for its broad use in printed circuit board manufacturing (as a final finish) and in corrosion protection.[12]
Synthesis
Although the synthetic methods to produce polyaniline are quite simple, the mechanism of polymerization is probably complex. The formation of leucoemeraldine can be described as follows, where [O] is a generic oxidant:[4]
- n C6H5NH2 + [O] → [C6H4NH]n + H2O
The most common oxidant is ammonium persulfate. The components are each dissolved in 1 M hydrochloric acid (other acids can be used), and the two solutions slowly combined. The reaction is very exothermic. The polymer precipitates as an unstable dispersion with micrometer-scale particulates.
(per)nigraniline is prepared by oxidation of the emeraldine base, one typical oxidant being meta-chloroperoxybenzoic acid:[13]
- {[C6H4NH]2[C6H4N]2}n + RCO3H → [C6H4N]n + H2O + RCO2H
Processing
The synthesis of polyaniline nanostructures is facile.[14]
Using special polymerisation procedures and surfactant dopants, the obtained polyaniline powder can be made dispersible and hence useful for practical applications. Bulk synthesis of polyaniline nanofibers has led to a highly scalable and commercially applicable form of polyaniline that has been researched extensively since their discovery in 2002.[15]
A multi-stage model for the formation of emeraldine base is proposed. In the first stage of the reaction the pernigraniline PS salt oxidation state is formed. In the second stage pernigraniline is reduced to the emeraldine salt as aniline monomer gets oxidized to the radical cation.[8] In the third stage this radical cation couples with ES salt. This process can be followed by light scattering analysis which allows the determination of the absolute molar mass. According to one study in the first step a DP of 265 is reached with the DP of the final polymer at 319. Approximately 19% of the final polymer is made up of the aniline radical cation which is formed during the reaction.[16]
Polyaniline is typically produced in the form of long-chain polymer aggregates, surfactant (or dopant) stabilized nanoparticle dispersions, or stabilizer-free nanofiber dispersions depending on the supplier and synthetic route. Surfactant or dopant stabilized polyaniline dispersions have been available for commercial sale since the late 1990s.[12]
Applications
Polyaniline and the other conducting polymers such as polythiophene, polypyrrole, and PEDOT/PSS have potential for applications due to their light weight, conductivity, mechanical flexibility and low cost. Polyaniline is especially attractive because it is relatively inexpensive, has three distinct oxidation states with different colors and has an acid/base doping response. This latter property makes polyaniline an attractive for acid/base chemical vapor sensors, supercapacitors and biosensors. The different colors, charges and conformations of the multiple oxidation states also make the material promising for applications such as actuators, supercapacitors and electrochromics. They are suitable for manufacture of electrically conducting yarns, antistatic coatings, electromagnetic shielding, and flexible electrodes.
Attractive fields for current and potential utilization of polyaniline is in antistatics, charge dissipation or electrostatic dispersive (ESD) coatings and blends, electromagnetic interference shielding (EMI), anticorrosive coatings, hole injection layers,[17] transparent conductors, indium tin oxide replacements, actuators, chemical vapor and solution based sensors, electrochromic coatings (for color change windows, mirrors etc.), PEDOT-PSS replacements, toxic metal recovery, catalysis, fuel cells and active electronic components such as for non-volatile memory.
Currently, the major applications are printed circuit board manufacturing (final finishes, used in millions of m² every year), antistatic and ESD coatings, and corrosion protection.
References
- ↑ Okamoto, Yoshikuko and Brenner, Walter (1964) "Polymers", Ch. 7 pp. 125–158 in Organic Semiconductors, Reinhold.
- 1 2 MacDiarmid, Alan G. (2001). ""Synthetic Metals": A Novel Role for Organic Polymers (Nobel Lecture)". Angewandte Chemie International Edition. 40 (14): 2581. doi:10.1002/1521-3773(20010716)40:14<2581::AID-ANIE2581>3.0.CO;2-2.
- ↑ Letheby, H. (1862). "XXIX.-On the production of a blue substance by the electrolysis of sulphate of aniline". Journal of the Chemical Society. 15: 161. doi:10.1039/JS8621500161.
- 1 2 Chiang, J.C.; MacDiarmid, A. G. (1986). "'Polyaniline': Protonic Acid Doping of the Emeraldine Form to the Metallic Regime". Synthetic Metals. 1 (13): 193. doi:10.1016/0379-6779(86)90070-6.
- ↑ Heeger, Alan (2001). "Nobel Lecture: Semiconducting and metallic polymers: The fourth generation of polymeric materials". Reviews of Modern Physics. 73 (3): 681. Bibcode:2001RvMP...73..681H. doi:10.1103/RevModPhys.73.681.
- ↑ Tzamalis, G.; Zaidi, N.; Monkman, A. (2003). "Applicability of the localization-interaction model to magnetoconductivity studies of polyaniline films at the metal-insulator boundary". Physical Review B. 68 (24): 245106. Bibcode:2003PhRvB..68x5106T. doi:10.1103/PhysRevB.68.245106.
- ↑ Cattena, Carlos J.; Bustos-Marún, Raúl A.; Pastawski, Horacio M. (2010). "Crucial role of decoherence for electronic transport in molecular wires: Polyaniline as a case study". Physical Review B. 82 (14): 144201. Bibcode:2010PhRvB..82n4201C. doi:10.1103/PhysRevB.82.144201.
- 1 2 Feast, W.J.; Tsibouklis, J.; Pouwer, K.L.; Groenendaal, L.; Meijer, E.W. (1996). "Synthesis, processing and material properties of conjugated polymers". Polymer. 37 (22): 5017. doi:10.1016/0032-3861(96)00439-9.
- ↑ Huang, Li-Ming; Chen, Cheng-Hou; Wen, Ten-Chin (2006). "Development and characterization of flexible electrochromic devices based on polyaniline and poly(3,4-ethylenedioxythiophene)-poly(styrene sulfonic acid)". Electrochimica Acta. 51 (26): 5858. doi:10.1016/j.electacta.2006.03.031.
- ↑ Virji, Shabnam; Huang, Jiaxing; Kaner, Richard B.; Weiller, Bruce H. (2004). "Polyaniline Nanofiber Gas Sensors: Examination of Response Mechanisms". Nano Letters. 4 (3): 491. Bibcode:2004NanoL...4..491V. doi:10.1021/nl035122e.
- ↑ Hammo, Shamil M. (2012). "Effect of Acidic Dopants properties on the Electrical Conductivity of Poly aniline". Tikrit Journal of Pure Science. 17 (2).
- 1 2 Wessling, Bernhard (2010). "New Insight into Organic Metal Polyaniline Morphology and Structure". Polymers. 2 (4): 786. doi:10.3390/polym2040786.
- ↑ Sapurina, I. Yu. and Shishov, M. A. (2012) "Oxidative Polymerization of Aniline: Molecular Synthesis of Polyaniline and the Formation of Supramolecular Structures", in New Polymers for Special Applications, Ailton De Souza Gomes (Ed.), ISBN 978-953-51-0744-6, InTech, doi:10.5772/48758.
- ↑ Ćirić-Marjanović, G. Polyaniline Nanostructures, in Nanostructured Conductive Polymers (ed A. Eftekhari), 2010, John Wiley & Sons, Ltd, Chichester, UK. doi:10.1002/9780470661338.ch2 PDF
- ↑ Huang, Jiaxing; Virji, Shabnam; Weiller, Bruce H.; Kaner, Richard B. (2003). "Polyaniline Nanofibers: Facile Synthesis and Chemical Sensors" (PDF). Journal of the American Chemical Society. 125 (2): 314–5. doi:10.1021/ja028371y. PMID 12517126. Archived from the original (PDF) on July 22, 2010.
- ↑ Kolla, Harsha S.; Surwade, Sumedh P.; Zhang, Xinyu; MacDiarmid, Alan G.; Manohar, Sanjeev K. (2005). "Absolute Molecular Weight of Polyaniline". Journal of the American Chemical Society. 127 (48): 16770–1. doi:10.1021/ja055327k. PMID 16316207.
- ↑ Fehse, Karsten; Schwartz, Gregor; Walzer, Karsten; Leo, Karl (2007). "Combination of a polyaniline anode and doped charge transport layers for high-efficiency organic light emitting diodes". Journal of Applied Physics. 101 (12): 124509. Bibcode:2007JAP...101l4509F. doi:10.1063/1.2748864.