Annonacin
 | |
| Names | |
|---|---|
| IUPAC name
(5S)-5-Methyl-3-[(2R,8R,13R)-2,8,13-trihydroxy-13- [(2R,5R)-5-[(1R)-1- hydroxytridecyl]-2-tetrahydrofuranyl] tridecyl]-5H-furan-2-one | |
| Identifiers | |
| 111035-65-5 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEMBL | ChEMBL476498 |
| ChemSpider | 314587 |
| PubChem | 354398 |
| |
| |
| Properties | |
| C35H64O7 | |
| Molar mass | 596.89 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Annonacin is a neurotoxic chemical compound found in some fruits such as the paw paw (custard apple), soursop, and others from the family Annonaceae. It is a member of the class of compounds known as acetogenins.
Neurotoxicity
Annonacin is a neurotoxin[1][2][3][4] that is believed to be responsible for the incidence of atypical parkinsonism in the Caribbean island of Guadeloupe where consumption of soursop and pawpaw is common.[5] Studies in rodents indicates that consumption of annonacin (3.8 and 7.6 mg per kg per day for 28 days) caused brain lesions consistent with Parkinson's disease.[6][7]
Along with other acetogenins, annonacin blocks mitochondrial complex I (NADH-dehydrogenase), which is responsible for the conversion of NADH to NAD+ and the build-up of a proton gradient over the mitochondrial inner membrane. This effectively disables a cell's ability to generate ATP via an oxidative pathway, ultimately forcing a cell into apoptosis or necrosis.[8]
Biosynthesis
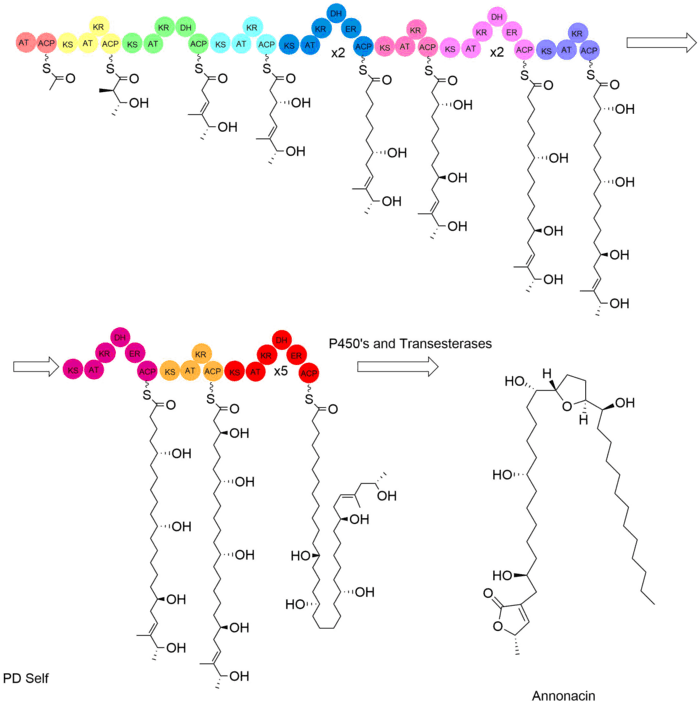
Based on basic polyketide synthesis, the biosynthesis of annonacin is likely accomplished by a modular polyketide synthase (PKS). Not much is known other than this about the domains responsible for the biosynthesis annonacin. The biosynthesis likely involves the use of 17 modules consisting of a number of enzymes commonly found in PKS. These include the acyl carrier protein (ACP), acetyl transferase (AT), ketosynthase (KS), malonyl transferase (MT; which can come carrying a variety of functionalities), ketoreductase (KR), dehydratase (DH), and enoyl reductase (ER). An example of the possible modular biosynthetic pathway detailing the combination of these enzymes and the subsequent modules can be seen in the figure below.
References
- ↑ Victor R. Preedy; Ronald Ross Watson; Vinood B. Patel, eds. (2011). Nuts and Seeds in Health and Disease Prevention. Academic Press. pp. 434–435.
- ↑ Robert A. Levine, Kristy M. Richards, Kevin Tran, Rensheng Luo, Andrew L. Thomas, and Robert E. Smith (2015). "Determination of Neurotoxic Acetogenins in Pawpaw (Asimina triloba) Fruit by LC-HRMS". J. Agric. Food Chem. 63 (4): 1053–1056. doi:10.1021/jf504500g.
- ↑ Potts, L. F.; Luzzio, F. A.; Smith, S. C.; Hetman, M; Champy, P; Litvan, I (2012). "Annonacin in Asimina triloba fruit: Implication for neurotoxicity". Neurotoxicology. 33 (1): 53–8. doi:10.1016/j.neuro.2011.10.009. PMID 22130466.
- ↑ Le Ven, J.; Schmitz-Afonso, I.; Touboul, D.; Buisson, D.; Akagah, B.; Cresteil, T.; Lewin, G.; Champy, P. (2011). "Annonaceae fruits and parkinsonism risk: Metabolisation study of annonacin, a model neurotoxin; evaluation of human exposure". Toxicology Letters. 205: S50. doi:10.1016/j.toxlet.2011.05.197.
- ↑ Annie Lannuzel, Merle Ruberg, and Patrick P. Michel (2008). "Atypical parkinsonism in the Caribbean island of Guadeloupe: Etiological role of the mitochondrial complex I inhibitor annonacin". Movement Disorders. 23 (15): 2122–2128. doi:10.1002/mds.22300.
- ↑ Lannuzel, A.; Michel, P. P.; Höglinger, G. U.; Champy, P.; Jousset, A.; Medja, F.; Lombès, A.; Darios, F.; et al. (2003). "The Mitochondrial Complex I Inhibitor Annonacin is Toxic to Mesencephalic Dopaminergic Neurons by Impairment of Energy Metabolism". Neuroscience. 121 (2): 287–296. doi:10.1016/S0306-4522(03)00441-X. PMID 14521988.
- ↑ Champy, P.; Höglinger, G. U.; Féger, J.; Gleye, C.; Hocquemiller, R.; Laurens, A.; Guérineau, V.; Laprévote, O.; et al. (2003). "Annonacin, a Lipophilic Inhibitor of Mitochondrial Complex I, induces Nigral and Striatal Neurodegeneration in Rats: Possible Relevance for Atypical Parkinsonism in Guadeloupe". Journal of Neurochemistry. 88 (1): 63–69. doi:10.1046/j.1471-4159.2003.02138.x. PMID 14675150.
- ↑ McLaughlin, J. L. (2008). "Paw Paw and Cancer: Annonaceous Acetogenins from Discovery to Commercial Products". Journal of Natural Products. 71 (7): 1311–1321. doi:10.1021/np800191t. PMID 18598079.
External links
- Pomper, K. W.; Lowe, J. D.; Crabtree, S. B.; Keller, W. (2009). "Identification of Annonaceous Acetogenins in the Ripe Fruit of the North American Pawpaw (Asimina triloba)" (PDF). Journal of Agricultural and Food Chemistry. 57 (18): 8339–8343. doi:10.1021/jf9018239. PMID 19711911. Archived from the original (pdf) on 2012-06-19.