Antipain
This article is about the protease inhbitor. For the article about pain relief, see analgesic.
 | |
| Names | |
|---|---|
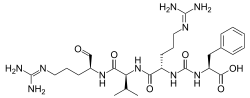
| IUPAC name
N2-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}-N5-(diaminomethylidene)-L-ornithyl-N-{(2S)-5-[(diaminomethylidene)amino]-1-oxopentan-2-yl}-L-valinamide | |
| Identifiers | |
| 37691-11-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 34678 |
| ECHA InfoCard | 100.048.738 |
| PubChem | 37817 |
| UNII | 47V479BE6L |
| |
| |
| Properties | |
| C27H44N10O6 | |
| Molar mass | 604.71 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Antipain is an oligopeptide isolated from actinomycetes and used in biochemical research as a protease inhibitor, reported in 1972 as the first natural peptide containing a ureylene group.[1] Specifically, it is an inhibitor of trypsin and papain.[2]
It has been crystallised in complex with carboxypeptidase from wheat[3] and Leishmania major oligopeptidase B.[4] In both cases the backbone carbonyl of the terminal arginine of antipain forms a covalent bond to the active site serine in the protease.
References
- ↑ Umezawa, S; Tatsuta, K; Fujimoto, K; Tsuchiya, T; Umezawa, H (1972). "Structure of antipain, a new Sakaguchi-positive product of streptomyces". The Journal of antibiotics. 25 (4): 267–70. doi:10.7164/antibiotics.25.267. PMID 5052959.
- ↑ Suda, H; Aoyagi, T; Hamada, M; Takeuchi, T; Umezawa, H (1972). "Antipain, a new protease inhibitor isolated from actinomycetes". The Journal of antibiotics. 25 (4): 263–6. doi:10.7164/antibiotics.25.263. PMID 4559651.
- ↑ PDB ENTRY 1bcr "Peptide aldehyde complexes with wheat serine carboxypeptidase II: implications for the catalytic mechanism and substrate specificity.". J.Mol.Biol. 225 (5): 714–25. 1996. PMID 8636973.
- ↑ PDB ENTRY 2xe4 "Crystal Structure of Leishmania Major Oligopeptidase B Gives Insight Into the Enzymatic Properties of a Trypanosomatid Virulence Factor.". J.Biol. Chem. 285 (50): 39249–59. 2010. doi:10.1074/jbc.M110.156679. PMC 2998157
 . PMID 20926390.
. PMID 20926390.
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.