Barbier reaction
| Barbier reaction | |
|---|---|
| Named after | Philippe Barbier |
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000084 |

The Barbier reaction is an organic reaction between an alkyl halide and a carbonyl group as an electrophilic substrate in the presence of magnesium, aluminium, zinc, indium, tin or its salts. The reaction product is a primary, secondary or tertiary alcohol. The reaction is similar to the Grignard reaction but the crucial difference is that the Barbier reaction is a one-pot synthesis whereas a Grignard reagent is prepared separately before addition of the carbonyl compound.[1] Barbier reactions are nucleophilic addition reactions that usually take place with relatively inexpensive and water insensitive metals or metal compounds in contrast to Grignard reagents or organolithium reagents. For this reason it is possible in many cases to run the reaction in water which makes the procedure part of green chemistry. The Barbier reaction is named after Victor Grignard's teacher Philippe Barbier.
Scope
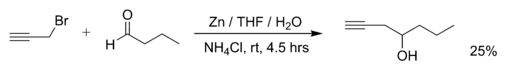
Examples of Barbier reactions are the reaction of propargylic bromide with butanal with zinc metal in water:[2]
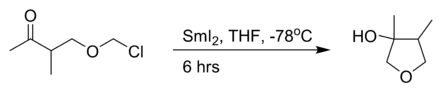
the intramolecular Barbier reaction with samarium(II) iodide:[3]
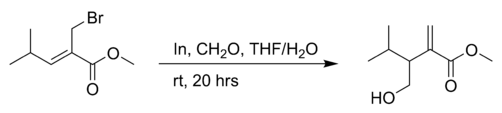
the reaction of an allyl bromide with formaldehyde in THF with indium powder:[4]
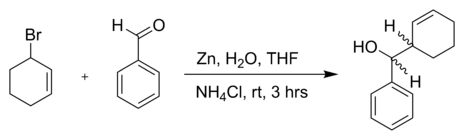
or another allyl bromide in a reaction with benzaldehyde and zinc powder in water:[5]
Asymmetric Variants
The synthesis of (+)-aspicillin, starts first with a hydroboration, then transmetallation to zinc which can then do an addition into the aldehyde substituent.[6]
-aspicillin.png)
See also
External links
- Barbier reaction @ University of Connecticut Website
References
- ↑ Barbier, P. (1899). "Synthèse du diéthylhepténol". Compt. Rend. 128: 110.
- ↑ Artur Jõgi & Uno Mäeorg (2001). "Zn Mediated Regioselective Barbier Reaction of Propargylic Bromides in THF/aq. NH4Cl Solution" (PDF). Molecules. 6 (12): 964–968. doi:10.3390/61200964. ISSN 1420-3049.
- ↑ Tore Skjæret & Tore Benneche (2001). "Preparation of oxo-substituted α-chloro ethers and their reaction with samarium diiodide". Arkivoc: KU–242A.
- ↑ George D. Bennett and Leo A. Paquette. "Methyl 3-(hydroxymethyl)-4-methyl-2-methylenepentanoate". Org. Synth.; Coll. Vol., 10, p. 77
- ↑ Gary W. Breton; John H. Shugart; Christine A. Hughey; Brian P. Conrad; Suzanne M. Perala (2001). "Use of Cyclic Allylic Bromides in the Zinc–Mediated Aqueous Barbier–Grignard Reaction" (PDF). Molecules. 6 (8): 655–662. doi:10.3390/60800655.
- ↑ De Brabander,J;et al. Tetrahedron Letters, 1995, Vol. 36, No. 15, pp. 2607-2610