Benperidol
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | N05AD07 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
983-42-6 |
| PubChem (CID) | 16363 |
| ChemSpider |
15521 |
| UNII |
97O6X78C53 |
| ChEMBL |
CHEMBL297302 |
| Chemical and physical data | |
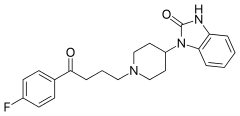
| Formula | C22H24FN3O2 |
| Molar mass | 381.443 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Benperidol is a drug which is a highly potent butyrophenone derivative. It is the most potent neuroleptic on the European market, with chlorpromazine equivalency as high as 75 to 100 (about 150 to 200% potency in terms of dose compared to haloperidol).[1] It is an antipsychotic, which can be used for the treatment of schizophrenia,[2] but it is primarily used to control antisocial hypersexual behaviour,[3] and is sometimes prescribed to sex offenders as a condition of their parole, as an alternative to anti-androgen drugs such as cyproterone acetate.[4]
Benperidol was discovered at Janssen Pharmaceutica in 1961.
Synthesis
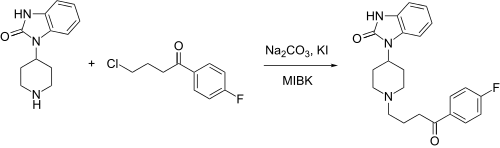
Benperidol synthesis:[5]
See also
- Timiperone has the same structure but thiourea instead of normal urea.
- Pimozide & Oxiperomide & Neflumozide are also made from 4-(1-Benzimidazolinone)piperidine prec.
- Droperidol is self-same albeit with a tetrahydropyridine ring.
References
- ↑ Möller; Müller; Bandelow: Neuroleptika, 2001, WVG; ISBN 3-8047-1773-X (in German)
- ↑ Bobon J, Collard J, Lecoq R, Benperidol and promazine: a "double blind" comparative study in mental geriatrics, Acta Neurol Belg. 1963 Oct;63:839-43.
- ↑ British National Formulary (49th), British Medical Association 2005 p 183
- ↑ Murray MA, Bancroft JH, Anderson DC, Tennent TG, Carr PJ., Endocrine changes in male sexual deviants after treatment with anti-androgens, oestrogens or tranquillizers, Journal of Endocrinology. 1975 Nov;67(2):179-88.
- ↑ BE 626307 (1963 to Janssen), C.A. 60, 10690c (1964), corresp. to GB 989755.
This article is issued from Wikipedia - version of the 6/25/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.