Benzopyran
 | |
| Identifiers | |
|---|---|
| 254-04-6 | |
| ChemSpider | 10651828 |
| |
| Properties | |
| C9H8O | |
| Molar mass | 132.16 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Benzopyran is an polycyclic organic compound that results from the fusion of a benzene ring to a heterocyclic pyran ring. According to current IUPAC nomenclature, the name chromene used in previous recommendations is retained; however, systematic ‘benzo’ names, for example 2H-1-benzopyran, are preferred IUPAC names for chromene, isochromene, chromane, isochromane, and their chalcogen analogues.[1] There are two isomers of benzopyran that vary by the orientation of the fusion of the two rings compared to the oxygen, resulting in 1-benzopyran (chromene) and 2-benzopyran (isochromene)—the number denotes where the oxygen atom is located by standard naphthalene-like nomenclature.
The radical form of benzopyran is paramagnetic. The unpaired electron is delocalized over the whole benzopyran molecule, rendering it less reactive than one would expect otherwise, a similar example is the cyclopentadienyl radical. Commonly, benzopyran is encountered in the reduced state, in which it is partially saturated with one hydrogen atom, introducing a tetrahedral CH2 group in the pyran ring. Therefore, there are many structural isomers owing to the multiple possible positions of the oxygen atom and the tetrahedral carbon atom:
 2H-chromene (2H-1-benzopyran) |
 4H-chromene (4H-1-benzopyran) |
| |
|
| |
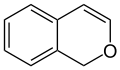 1H-isochromene (1H-2-benzopyran) |
 3H-isochromene (3H-2-benzopyran) |
See also
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. pp. xxxv, 211, 214. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.