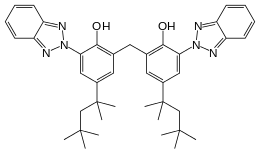
Bisoctrizole
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,2′-methanediylbis[6-(2H-benzotriazol-2-yl)-4-(2,4,4-trimethylpentan-2-yl)phenol] | |
| Identifiers | |
| 103597-45-1 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL2104957 |
| ChemSpider | 2808671 |
| ECHA InfoCard | 100.100.550 |
| PubChem | 3571576 |
| UNII | 8NT850T0YS |
| |
| |
| Properties | |
| C41H50N6O2 | |
| Molar mass | 658.88 g/mol |
| Melting point | 195.7 °C (384.3 °F; 468.8 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Bisoctrizole (INN[1][2]/USAN,[3] marketed by BASF as Tinosorb M and by MPI as Milestab 360, INCI methylene bis-benzotriazolyl tetramethylbutylphenol) is a benzotriazole-based organic compound that is added to sunscreens to absorb UV rays.
Bisoctrizole is a broad-spectrum ultraviolet radiation absorber, absorbing UVB as well as UVA rays. It also reflects and scatters UV. Bisoctrizole is a hybrid UV absorber, the only organic UV filter produced and microfine organic particles (< 200 nm),[4][5] like microfine zinc oxide and titanium dioxide. Where other organic UV absorbers need to be dissolved in either the oil or water phase, bisoctrizole dissolves poorly in both.
Bisoctrizole is added to the water phase of a sunscreen as a 50% suspension, whereas mineral micropigments are usually added to the oil phase. The bisoctrizole particles are stabilized by the surfactant decyl glucoside.
Bisoctrizole shows very little photodegradation and has a stabilizing effect on other UV absorbers, octyl methoxycinnamate (octinoxate) in particular.
When formulated into a sunscreen, bisoctrizole has minimal skin penetration.[6] Unlike some other organic sunscreen actives, it shows no estrogenic effects in vitro.[7]
Bisoctrizole is not approved by the U.S. Food and Drug Administration (FDA), but is approved in the EU and other parts of the world.[8][9][10]
References
- ↑ http://whqlibdoc.who.int/druginfo/18_4_2004_INN92.pdf
- ↑ http://whqlibdoc.who.int/druginfo/INN_2005_list54.pdf
- ↑ http://www.ama-assn.org/ama1/pub/upload/mm/365/bisoctrizol.doc
- ↑ Ciba TINOSORB M
- ↑ Herzog, B.; Mongiat, S.; Deshayes, C.; Neuhaus, M.; Sommer, K.; Mantler, A. (2002). "In vivo and in vitro assessment of UVA protection by sunscreen formulations containing either butyl methoxy dibenzoyl methane, methylene bis-benzotriazolyl tetramethylbutylphenol, or microfine ZnO". International Journal of Cosmetic Science. 24 (3): 170–85. doi:10.1046/j.1467-2494.2002.00137.x. PMID 18498509.
- ↑ Mavon A, Miquel C, Lejeune O, Payre B, Moretto P (2007). "In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen". Skin Pharmacol Physiol. 20 (1): 10–20. doi:10.1159/000096167. PMID 17035717.
- ↑ Ashby J, Tinwell H, Plautz J, Twomey K, Lefevre PA (December 2001). "Lack of binding to isolated estrogen or androgen receptors, and inactivity in the immature rat uterotrophic assay, of the ultraviolet sunscreen filters Tinosorb M-active and Tinosorb S". Regul Toxicol Pharmacol. 34 (3): 287–91. doi:10.1006/rtph.2001.1511. PMID 11754532.
- ↑ Manage Account - Modern Medicine
- ↑ CL1976L0768EN0150010.0001 1..107
- ↑ Australian Regulatory Guidelines for OTC Medicines - Chapter 10
External links
- http://www.cibasc.com/index/ind-index/ind-per_car/ind-pc-uv/ind-pc-uv-tinosorbm.htm
- http://www.dermatologytimes.com/dermatologytimes/article/articleDetail.jsp?id=159652
- http://pubs.acs.org/cen/coverstory/83/8315sunscreens.html
- http://www.fda.gov/ohrms/dockets/dockets/05n0446/05n-0446-bkg0001-03-Tab-01-vol2.pdf