Bouveault–Blanc reduction
| Bouveault-Blanc reduction | |
|---|---|
| Named after | Louis Bouveault Gustave Louis Blanc |
| Reaction type | Organic redox reaction |
| Identifiers | |
| Organic Chemistry Portal | bouveault-blanc-reduction |
| RSC ontology ID | RXNO:0000119 |
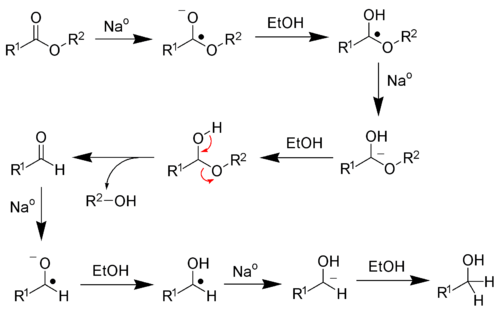
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal.[1][2][3][4]
This reaction is an inexpensive and large-scale alternative to lithium aluminium hydride reduction of esters.
Reaction mechanism
Sodium metal is a single-electron reducing agent, meaning the sodium metal will transfer electrons one at a time. Four sodium atoms are required to fully reduce each ester to alcohols. Ethanol serves as a proton source.
See also
- Acyloin condensation - The reductive coupling of esters, using sodium, to yield an α-hydroxyketone
- Akabori amino-acid reaction - The reduction of amino acid esters, by sodium, to yield aldehydes
- Birch reduction - For the reduction of alkenes using sodium
- Bouveault aldehyde synthesis - Another organometallic reaction by Bouveault where a Grignard reagent is converted to an aldehyde
References
- ↑ Bouveault, L.; Blanc, G. (1903). "Préparation des alcools primaires au moyen des acides correspondants" [Preparation of primary alcohols by means of the corresponding acids]. Compt. Rend. 136: 1676–1678.
- ↑ Bouveault, L.; Blanc, G. (1903). "Préparation des alcools primaires au moyen des acides correspondants" [Preparation of primary alcohols by means of the corresponding acids]. Compt. Rend. 137: 60–62.
- ↑ Bouveault, L.; Blanc, G. (1904). "Transformation des acides monobasiques saturés dans les alcools primaires correspondants" [Transforming saturated monobasic acids into the corresponding primary alcohols]. Bull. Soc. Chim. France. 31: 666–672.
- ↑ Adkins, H.; Gillespie, R. H. (1955). "Oleyl alcohol". Org. Synth.; Coll. Vol., 3, p. 671
External links
This article is issued from Wikipedia - version of the 7/31/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.