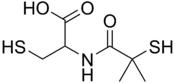
Bucillamine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-[(2-Methyl-2-sulfanylpropanoyl)amino]-3-sulfanylpropanoic acid | |
| Identifiers | |
| 65002-17-7 R | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEMBL | ChEMBL80830 |
| ChemSpider | 2365 |
| KEGG | D01809 |
| MeSH | bucillamine |
| PubChem | 2459 656604 R 636373 S |
| UNII | R80LRA5WTF |
| |
| |
| Properties | |
| C7H13NO3S2 | |
| Molar mass | 223.31 g·mol−1 |
| log P | 1.032 |
| Acidity (pKa) | 3.012 |
| Basicity (pKb) | 10.985 |
| Pharmacology | |
| M01CC02 (WHO) | |
| Related compounds | |
| Related alkanoic acids |
|
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bucillamine is an antirheumatic agent developed from tiopronin. It is mainly used in Japan and Korea. Activity is mediated by the two thiol groups that the molecule contains. Research done in USA showed positive transplant preservation properties.[1]
References
- ↑ Amersi, F.; Nelson, S. K.; Shen, X. D.; Kato, H.; Melinek, J.; Kupiec-Weglinski, J. W.; Horwitz, L. D.; Busuttil, R. W.; Horwitz, M. A. (2002). "Bucillamine, a Thiol Antioxidant, Prevents Transplantation-Associated Reperfusion Injury" (pdf). Proceedings of the National Academy of Sciences. 99 (13): 8915–8920. doi:10.1073/pnas.132026099. PMC 124398
 . PMID 12084933.
. PMID 12084933.
This article is issued from Wikipedia - version of the 6/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.