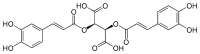
Cichoric acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3R)-2,3-bis{[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy}butanedioic acid | |
| Other names
2R,3R-O-dicaffeoyltartaric acid | |
| Identifiers | |
| 6537-80-0 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL282731 |
| ChemSpider | 4445078 |
| ECHA InfoCard | 100.164.046 |
| KEGG | C10437 |
| PubChem | 5281764 |
| |
| |
| Properties | |
| C22H18O12 | |
| Molar mass | 474.371 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Cichoric acid is a hydroxycinnamic acid, an organic compound of the phenylpropanoid class and occurs in a variety of plant species. It is a derivative of both caffeic acid and tartaric acid.[1]
As a suitable marker for the distinction of Echinacea species, it is often assayed using RP-HPLC and Thin layer chromatography (TLC) methods.[2]
Sources
Cichoric acid has first been isolated from Cichorium intybus (chicory) but also occurs in significant amounts in Echinacea, in particular E. purpurea, dandelion leaves, basil, lemon balm, and aquatic plants, including algae and seagrasses.[3][4][5][6]
Biological functions
Cichoric acid has been shown to stimulate phagocytosis in both in vitro and in vivo studies, to inhibit the function of hyaluronidase (an enzyme that breaks down hyaluronic acid in the human body), to protect collagen from damage due to free radicals, and to inhibit the function of HIV-1 integrase.[7][8]
See also
- Caftaric acid (Monocaffeyltartaric acid)
References
- ↑ John Shi; Giuseppe Mazza; Marc Le Maguer (27 February 2002). Functional Foods: Biochemical and Processing Aspects. CRC Press. pp. 241–. ISBN 978-1-4200-1287-3.
- ↑ Bauer R, Khan IA, Wagner H. Echinacea-Drogen, Standardisierung mittels HPLC und DC. Deutsche Apotheker Zeitung, 1986, 126:1065–1070. Citation in WHO Monographs on Selected Medicinal Plants - Volume 1
- ↑ I.D. Chkhikvishvili and G.I. Kahrebava (2001). "Cichoric and Chlorogenic Acids in Plant Species from Georgia". Applied Biochemistry and Microbiology, 37 (2): 188-191. doi:10.1023/a:1002888016985
- ↑ Lee J. (2010). "Caffeic acid derivatives in dried Lamiaceae and Echinacea purpurea products". Journal of Functional Foods 2, 158-162. doi|10.1016/j.jff.2010.02.003
- ↑ Lee J. Scagel, C.F. (2009). "Chicoric acid found in basil (Ocimum basilicum L.) leaves.". Food Chemistry 115, 650-656. doi:10.1016/j.foodchem.2008.12.075
- ↑ Lee J. Scagel, C.F. (2013). "Chicoric acid: chemistry, distribution, and production". Frontiers in Chemistry 1:40. doi: 10.3389/fchem.2013.00040 http://journal.frontiersin.org/article/10.3389/fchem.2013.00040/abstract#
- ↑ Mazza, G.; Oomah, B. Dave (2000), Herbs, Botanicals & Teas, CRC Press, p. 51, ISBN 1-56676-851-9, retrieved 2008-12-09
- ↑ Miller, Sandra Carol; Yu, He-Ci (2004), Echinacea, CRC Press, p. 140, ISBN 0-415-28828-2, retrieved 2008-12-09