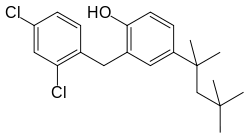
Clofoctol
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Rectal (suppository)[1] |
| ATC code | J01XX03 (WHO) |
| Pharmacokinetic data | |
| Bioavailability | 98%[1] |
| Metabolism | Hepatic glucuronidation[1] |
| Excretion | Biliary[1] |
| Identifiers | |
| |
| CAS Number |
37693-01-9 |
| PubChem (CID) | 2799 |
| ChemSpider |
2697 |
| UNII |
704083NI0R |
| KEGG |
D07244 |
| ChEMBL | CHEMBL1476605 |
| ECHA InfoCard | 100.048.739 |
| Chemical and physical data | |
| Formula | C21H26Cl2O |
| Molar mass | 365.336 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Clofoctol is a bacteriostatic antibiotic. It is used in the treatment of respiratory tract and ear, nose and throat infections caused by Gram-positive bacteria.[1] It is marketed in France under the trade name Octoplus and in Italy as Gramplus.
It is only functional against Gram-positive bacteria.[2]
It penetrates into human lung tissue.[3]
References
- 1 2 3 4 5 "Gramplus" (in Italian). Studio Medico Torrino. July 25, 2007. Retrieved 2007-08-10.
- ↑ Combe J, Simonnet F, Yablonsky F, Simonnet G (1980). "[Clofoctol binding by the bacteria (author's transl)]". J Pharmacol (in French). 11 (4): 411–25. PMID 6782374.
- ↑ Danesi R, Gasperini M, Senesi S, Freer G, Angeletti CA, Del Tacca M (1988). "A pharmacokinetic study of clofoctol in human plasma and lung tissue by using a microbiological assay". Drugs Exp Clin Res. 14 (1): 39–43. PMID 3391105.
This article is issued from Wikipedia - version of the 5/24/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.