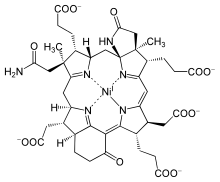
Cofactor F430
 | |
| Identifiers | |
|---|---|
| 73145-13-8 | |
| PubChem | 5460020 |
| Properties | |
| C 42H 51N 6NiO– 13 | |
| Molar mass | 906.58014 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
F430 is the prosthetic group of the enzyme methyl coenzyme M reductase.[1] This enzyme catalyzes the release of methane in the final step of methanogenesis:
It is found only in methanogenic Archaea.[2]
Corphin in context
Nature uses multiple tetrapyrroles - hemes, chlorophyll, and cobalamin. Of the tetrapyrroles with 5 double bonds, F430 is the most reduced. This particular tetrapyrrole derivative is called a corphin. Because of its relative lack of conjugated unsaturation, it is yellow, not the intense purple-red associated with more unsaturated tetrapyrroles. It is also the only tetrapyrrole derivative found in nature to contain nickel. Ni(II) is too small for the N4 binding site of the corphin, which causes the macrocycle to adopt a ruffled structure. Its structure was deduced by X-ray crystallography and NMR spectroscopy.[3]
F430 occurs in particularly high concentrations in archaea that are thought to be involved in reverse methanogenesis. Organisms that promote this remarkable reaction contain 7% by weight nickel protein.[4]
References
- ↑ Stephen W., Ragdale (2014). "Chapter 6. Biochemistry of Methyl-Coenzyme M Reductase: The Nickel Metalloenzyme that Catalyzes the Final Step in Synthesis and the First Step in Anaerobic Oxidation of the Greenhouse Gas Methane". In Peter M.H. Kroneck and Martha E. Sosa Torres. The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. 14. Springer. pp. 125–145. doi:10.1007/978-94-017-9269-1_6.
- ↑ Thauer RK (1998). "Biochemistry of Methanogenesis: a Tribute to Marjory Stephenson". Microbiology. 144 (9): 2377–2406. doi:10.1099/00221287-144-9-2377. PMID 9782487.
- ↑ Färber G, Keller W, Kratky C, Jaun B, Pfaltz A, Spinner C, Kobelt A, Eschenmoser A (1991). "Coenzyme F430 from Methanogenic Bacteria : Complete Assignment of Configuration Based on an X-ray Analysis of 12,13-diepi-F430 Pentamethyl Ester and on NMR Spectroscopy". Helvetica Chimica Acta. 74: 697–716. doi:10.1002/hlca.19910740404.
- ↑ Krüger M, Meyerdierks A, Glöckner FO, et al. (December 2003). "A conspicuous nickel protein in microbial mats that oxidize methane anaerobically". Nature. 426 (6968): 878–81. doi:10.1038/nature02207. PMID 14685246.