Balsalazide
 | |
| Clinical data | |
|---|---|
| Trade names | Colazal, Giazo |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a699052 |
| Pregnancy category |
|
| ATC code | A07EC04 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | <1% |
| Protein binding | ≥99% |
| Biological half-life | 12hr |
| Identifiers | |
| |
| CAS Number |
80573-04-2 |
| PubChem (CID) | 5362070 |
| DrugBank |
DB01014 |
| ChemSpider |
10662422 |
| UNII |
P80AL8J7ZP |
| ChEBI |
CHEBI:267413 |
| ChEMBL |
CHEMBL1201346 |
| Chemical and physical data | |
| Formula | C17H15N3O6 |
| Molar mass | 357.318 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Balsalazide is an anti-inflammatory drug used in the treatment of inflammatory bowel disease. It is sold under the brand names Giazo, Colazal in the US and Colazide in the UK. It is also sold in generic form in the US by several generic manufacturers.
It is usually administered as the disodium salt. Balsalazide releases mesalazine, also known as 5-aminosalicylic acid, or 5-ASA,[1] in the large intestine. Its advantage over that drug in the treatment of ulcerative colitis is believed to be the delivery of the active agent past the small intestine to the large intestine, the active site of ulcerative colitis.
Synthesis
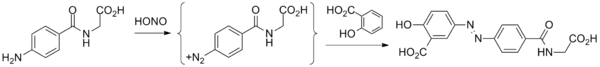
Balsalazide synthesis: Biorex Laboratories, GB 2080796 (1986).
- Starting material is 4-aminohippuric acid, obtained by coupling para-aminobenzoic acid and glycine.
- That product is then treated with nitrous acid to give the diazonium salt.
- Reaction of this species with salicylic acid proceeds at the position para to the phenol to give balsalazide.
References
- ↑ Kruis, W.; Schreiber, I.; Theuer, D.; Brandes, J. W.; Schütz, E.; Howaldt, S.; Krakamp, B.; Hämling, J.; Mönnikes, H.; Koop, I.; Stolte, M.; Pallant, D.; Ewald, U. (2001). "Low dose balsalazide (1.5 g twice daily) and mesalazine (0.5 g three times daily) maintained remission of ulcerative colitis but high dose balsalazide (3.0 g twice daily) was superior in preventing relapses". Gut. 49 (6): 783–789. doi:10.1136/gut.49.6.783. PMC 1728533
 . PMID 11709512.
. PMID 11709512.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.