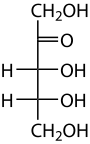
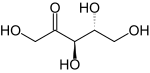
Ribulose
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(3R,4R)-1,3,4,5-Tetrahydroxypentan-2-one | |||
| Other names
D-erythro-2-Pentulose Adonose Arabinulose Araboketose Ribosone | |||
| Identifiers | |||
| 488-84-6 (D) 2042027-5 (L) 5556-48-9 (DL) | |||
| 3D model (Jmol) | (D): Interactive image (L): Interactive image | ||
| ChEBI | CHEBI:28721 | ||
| ChemSpider | 133316 | ||
| ECHA InfoCard | 100.006.989 | ||
| PubChem | 151261 | ||
| |||
| |||
| Properties | |||
| C5H10O5 | |||
| Molar mass | 150.13 g·mol−1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Ribulose is a ketopentose — a monosaccharide containing five carbon atoms, and including a ketone functional group. It has chemical formula C5H10O5. Two enantiomers are possible, D-ribulose (D-erythro-pentulose) and L-ribulose (L-erythro-pentulose). D-Ribulose is the diastereomer of D-xylulose.
Ribulose sugars are composed in the pentose phosphate pathway. They are important in the formation of many bioactive substances. For example, D-ribulose is an intermediate in the fungal pathway for D-arabitol production. Also, as the 1,5-bisphosphate, D-ribulose combines with carbon dioxide at the start of the photosynthesis process in green plants (carbon dioxide trap).
References
This article is issued from Wikipedia - version of the 11/13/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.