Zebrafish
| Danio rerio | |
|---|---|
 | |
| An adult female zebrafish | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Actinopterygii |
| Order: | Cypriniformes |
| Family: | Cyprinidae |
| Genus: | Danio |
| Species: | D. rerio |
| Binomial name | |
| Danio rerio (F. Hamilton, 1822) | |
| Synonyms | |
| |
The zebrafish (Danio rerio) is a tropical freshwater fish belonging to the minnow family (Cyprinidae) of the order Cypriniformes.[1] Native to the Himalayan region, it is a popular aquarium fish, frequently sold under the trade name zebra danio.[2] The zebrafish is also an important and widely used vertebrate model organism in scientific research, and was the first vertebrate to be cloned.[3] It is particularly notable for its regenerative abilities,[4] and has been modified by researchers to produce several transgenic strains.[5][6][7]
Taxonomy
The zebrafish is a derived member of the genus Danio, of the family Cyprinidae. It has a sister-group relationship with Danio kyathit.[8] Zebrafish are also closely related to the genus Devario, as demonstrated by a phylogenetic tree of close species.[9] The zebrafish was referred to in scientific literature as Brachydanio rerio for many years until its reassignment to the genus Danio.[10]
Distribution
The zebrafish is native to the streams of the southeastern Himalayan region,[8] and is found in parts of India, Pakistan, Bangladesh, Nepal, and Burma.[11] The species arose in the Ganges region in eastern India, and commonly inhabits streams, canals, ditches, ponds, and slow-moving or stagnant water bodies, including rice fields.[12] Zebrafish have been introduced to parts of the United States, presumably by deliberate release or by escape from fish farms.[11]
Description
The zebrafish is named for the five uniform, pigmented, horizontal, blue stripes on the side of the body, which are reminiscent of a zebra's stripes, and which extend to the end of the caudal fin. Its shape is fusiform and laterally compressed, with its mouth directed upwards. The male is torpedo-shaped, with gold stripes between the blue stripes; the female has a larger, whitish belly and silver stripes instead of gold. Adult females exhibit a small genital papilla in front of the anal fin origin. The zebrafish can grow to 6.4 cm (2.5 in) in length, although it seldom grows larger than 4 cm (1.6 in) in captivity. Its lifespan in captivity is around two to three years, although in ideal conditions, this may be extended to over five years.[12][13]
Reproduction
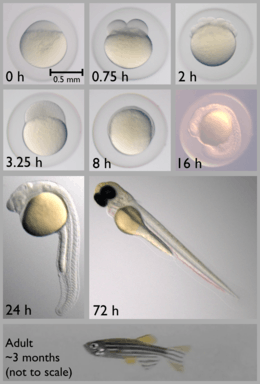
The approximate generation time for Danio rerio is three months. A male must be present for ovulation and spawning to occur. Females are able to spawn at intervals of two to three days, laying hundreds of eggs in each clutch. Upon release, embryonic development begins; absent sperm, growth stops after the first few cell divisions. Fertilized eggs almost immediately become transparent, a characteristic that makes D. rerio a convenient research model species.[12]
The zebrafish embryo develops rapidly, with precursors to all major organs appearing within 36 hours of fertilization. The embryo begins as a yolk with a single enormous cell on top (see image, 0 h panel), which divides into two (0.75 h panel) and continues dividing until there are thousands of small cells (3.25 h panel). The cells then migrate down the sides of the yolk (8 h panel) and begin forming a head and tail (16 h panel). The tail then grows and separates from the body (24 h panel). The yolk shrinks over time because the fish uses it for food as it matures during the first few days (72 h panel). After a few months, the adult fish reaches reproductive maturity (bottom panel).
To encourage the fish to spawn, some researchers use a fish tank with a sliding bottom insert, which reduces the depth of the pool to simulate the shore of a river. Zebrafish spawn best in the morning due to their Circadian rhythms. Researchers have been able to collect 10,000 embryos in 10 minutes using this method.[14] Male zebrafish are furthermore known to respond to more pronounced markings on females, i.e., "good stripes", but in a group, males will mate with whichever females they can find. What attracts females is not currently understood. The presence of plants, even plastic plants, also apparently encourages spawning.[14]
Feeding
Zebrafish are omnivorous, primarily eating zooplankton, phytoplankton, insects and insect larvae, although they can eat a variety of other foods, such as worms and small crustaceans, if their preferred food sources are not readily available.[12]
In the aquarium
Zebrafish are hardy fish and considered good for beginner aquarists. Their enduring popularity can be attributed to their playful disposition,[15] as well as their rapid breeding, aesthetics, cheap price and broad availability. They also do well in schools or shoals of six or more, and interact well with other fish species in the aquarium. However, they are susceptible to Oodinium or velvet disease, microsporidia (Pseudoloma neurophilia), and Mycobacterium species. Given the opportunity, adults eat hatchlings, which may be protected by separating the two groups with a net, breeding box or separate tank.
The Zebra Danio was also used to make genetically modified fish and were the first species to be sold as GloFish (fluorescent colored fish).
Strains
In late 2003, transgenic zebrafish that express green, red, and yellow fluorescent proteins became commercially available in the United States. The fluorescent strains are tradenamed GloFish; other cultivated varieties include "golden", "sandy", "longfin" and "leopard".
The leopard danio, previously known as Danio frankei, is a spotted colour morph of the zebrafish which arose due to a pigment mutation.[16] Xanthistic forms of both the zebra and leopard pattern, along with long-finned subspecies, have been obtained via selective breeding programs for the aquarium trade.[17]
Various transgenic and mutant strains of zebrafish were stored at the China Zebrafish Resource Center (CZRC),[18] a non-profit organization, which was jointly supported by the Ministry of Science and Technology of China and the Chinese Academy of Sciences.
Wild-type strains
The Zebrafish Information Network (ZFIN) provides up-to-date information about current known wild-type (WT) strains of D. rerio, some of which are listed below.[19]
|
|
|
Hybrids
Hybrids between different Danio species may be fertile: for example, between D. rerio and D. nigrofasciatus.[9]
In scientific research

D. rerio is a common and useful scientific model organism for studies of vertebrate development and gene function. Its use as a laboratory animal was pioneered by the American molecular biologist George Streisinger and his colleagues at the University of Oregon in the 1970s and 1980s; Streisinger's zebrafish clones were the first successful vertebrate clones created.[3] Its importance has been consolidated by successful large-scale forward genetic screens (commonly referred to as the Tübingen/Boston screens). The fish has a dedicated online database of genetic, genomic, and developmental information, the Zebrafish Information Network (ZFIN). D. rerio is also one of the few fish species to have been sent into space.
Research with D. rerio has yielded advances in the fields of developmental biology, oncology,[20] toxicology,[21][22] reproductive studies, teratology, genetics, neurobiology, environmental sciences, stem cell research and regenerative medicine,[23][24] and evolutionary theory.[9]
Model characteristics
As a model biological system, the zebrafish possesses numerous advantages for scientists. Its genome has been fully sequenced, and it has well-understood, easily observable and testable developmental behaviors. Its embryonic development is very rapid, and its embryos are relatively large, robust, and transparent, and able to develop outside their mother.[25] Furthermore, well-characterized mutant strains are readily available.
Other advantages include the species' nearly constant size during early development, which enables simple staining techniques to be used, and the fact that its two-celled embryo can be fused into a single cell to create a homozygous embryo. The zebrafish is also demonstrably similar to mammalian models and humans in toxicity testing, and exhibits a diurnal sleep cycle with similarities to mammalian sleep behavior.[26] However, zebrafish are not a universally ideal research model; there are a number of disadvantages to their scientific use, such as the absence of a standard diet[27] and the presence of small but important differences between zebrafish and mammals in the roles of some genes related to human disorders.[28][29]
Regeneration
Zebrafish have the ability to regenerate their fins, skin, heart, lateral line hair cells, and brain during their larval stages.[30][31] In 2011, the British Heart Foundation ran an advertising campaign publicising its intention to study the applicability of this ability to humans, stating that it aimed to raise £50 million in research funding.[32][33]
Zebrafish have also been found to regenerate photoreceptor cells and retinal neurons following injury, which has been shown to be mediated by the dedifferentiation and proliferation of Müller glia.[34] Researchers frequently amputate the dorsal and ventral tail fins and analyze their regrowth to test for mutations. It has been found that histone demethylation occurs at the site of the amputation, switching the zebrafish's cells to an "active", regenerative, stem cell-like state.[35] In 2012, Australian scientists published a study revealing that zebrafish use a specialised protein, known as fibroblast growth factor, to ensure their spinal cords heal without glial scarring after injury.[4] In addition, hair cells of the posterior lateral line have also been found to regenerate following damage or developmental disruption.[31][36] Study of gene expression during regeneration has allowed for the identification of several important signaling pathways involved in the process, such as Wnt signaling and Fibroblast growth factor.[36][37]
In probing disorders of the nervous system, including neurodegenerative diseases, movement disorders, psychiatric disorders and deafness, researchers are using the zebrafish to understand how the genetic defects underlying these conditions cause functional abnormalities in the human brain, spinal cord and sensory organs. Researchers have also studied the zebrafish to gain new insights into the complexities of human musculoskeletal diseases, such as muscular dystrophy.[38] Another focus of zebrafish research is to understand how a gene called Hedgehog, a biological signal that underlies a number of human cancers, controls cell growth.
Genetics
Gene expression
Due to their short lifecycles and relatively large clutch sizes, zebrafish are a useful model for genetic studies. A common reverse genetics technique is to reduce gene expression or modify splicing using Morpholino antisense technology. Morpholino oligonucleotides (MO) are stable, synthetic macromolecules that contain the same bases as DNA or RNA; by binding to complementary RNA sequences, they can reduce the expression of specific genes or block other processes from occurring on RNA. MO can be injected into one cell of an embryo after the 32-cell stage, reducing gene expression in only cells descended from that cell. However, cells in the early embryo (less than 32 cells) are interpermeable to large molecules,[39][40] allowing diffusion between cells.
A known problem with gene knockdowns is that, because the genome underwent a duplication after the divergence of ray-finned fishes and lobe-finned fishes, it is not always easy to silence the activity one of the two gene paralogs reliably due to complementation by the other paralog.[41] Despite the complications of the zebrafish genome, a number of commercially available global platforms exist for analysis of both gene expression by microarrays and promoter regulation using ChIP-on-chip.[42]
Genome sequencing
The Wellcome Trust Sanger Institute started the zebrafish genome sequencing project in 2001, and the full genome sequence of the Tuebingen reference strain is publicly available at the National Center for Biotechnology Information (NCBI)'s Zebrafish Genome Page. The zebrafish reference genome sequence is annotated as part of the Ensembl project, and is maintained by the Genome Reference Consortium.[43]
In 2009, researchers at the Institute of Genomics and Integrative Biology in Delhi, India, announced the sequencing of the genome of a wild zebrafish strain, containing an estimated 1.7 billion genetic letters.[44][45] The genome of the wild zebrafish was sequenced at 39-fold coverage. Comparative analysis with the zebrafish reference genome revealed over 5 million single nucleotide variations and over 1.6 million insertion deletion variations. The zebrafish reference genome sequence of 1.4GB and over 26,000 protein coding genes was published by Kerstin Howe et al. in 2013.[46]
Mitochondrial DNA
In October 2001, researchers from the University of Oklahoma published D. rerio's complete mitochondrial DNA sequence.[47] Its length is 16,596 base pairs. This is within 100 base pairs of other related species of fish, and it is notably only 18 pairs longer than the goldfish (Carassius auratus) and 21 longer than the carp (Cyprinus carpio). Its gene order and content are identical to the common vertebrate form of mitochondrial DNA. It contains 13 protein-coding genes and a noncoding control region containing the origin of replication for the heavy strand. In between a grouping of five tRNA genes, a sequence resembling vertebrate origin of light strand replication is found. It is difficult to draw evolutionary conclusions because it is difficult to determine whether base pair changes have adaptive significance via comparisons with other vertebrates' nucleotide sequences.[47]
Pigmentation genes
In 1999, the nacre mutation was identified in the zebrafish ortholog of the mammalian MITF transcription factor.[48] Mutations in human MITF result in eye defects and loss of pigment, a type of Waardenburg Syndrome. In December 2005, a study of the golden strain identified the gene responsible for its unusual pigmentation as SLC24A5, a solute carrier that appeared to be required for melanin production, and confirmed its function with a Morpholino knockdown. The orthologous gene was then characterized in humans and a one base pair difference was found to strongly segregate fair-skinned Europeans and dark-skinned Africans.[49]
Transgenesis
Transgenesis is a popular approach to study the function of genes in zebrafish. Construction of transgenic zebrafish is rather easy by a method using the Tol2 transposon system.[50]
Transparent adult bodies
In 2008, researchers at Boston Children's Hospital developed a new strain of zebrafish, named Casper, whose adult bodies had transparent skin.[6] This allows for detailed visualization of cellular activity, circulation, metastasis and many other phenomena. Because many gene functions are shared between fish and humans, the Casper strain is expected to yield insights into human diseases such as leukemia and other cancers.[6] In January 2013, Japanese scientists genetically modified a transparent zebrafish specimen to produce a visible glow during periods of intense brain activity, allowing the fish's "thoughts" to be recorded as specific regions of its brain lit up in response to external stimuli.[7]
Use in environmental monitoring
In January 2007, Chinese researchers at Fudan University genetically modified zebrafish to detect oestrogen pollution in lakes and rivers, which is linked to male infertility. The researchers cloned oestrogen-sensitive genes and injected them into the fertile eggs of zebrafish. The modified fish turned green if placed into water that was polluted by oestrogen.[5]
RNA Splicing
In 2015, researchers at Brown University discovered that 10% of zebrafish genes do not need to rely on the U2AF2 protein to initiate RNA splicing. These genes have the DNA base pairs AC and TG as repeated sequences at the ends of each intron. On the 3'ss (3' splicing site), the base pairs adenine and cytosine alternate and repeat, and on the 5'ss (5' splicing site), their complements thymine and guanine alternate and repeat as well. They found that there was less reliance on U2AF2 protein than in humans, in which the protein is required for the splicing process to occur. The pattern of repeating base pairs around introns that alters RNA secondary structure was found in other teleosts, but not in tetrapods. This indicates that an evolutionary change in tetrapods may have led to humans relying on the U2AF2 protein for RNA splicing while these genes in zebrafish undergo splicing regardless of the presence of the protein.[51]
Inbreeding depression
When close relatives mate, progeny may exhibit the detrimental effects of inbreeding depression. Inbreeding depression is predominantly caused by the homozygous expression of recessive deleterious alleles.[52] For zebra fish, inbreeding depression might be expected to be more severe in stressful environments, including those caused by anthropogenic pollution. Exposure of zebra fish to environmental stress induced by the chemical clotrimazole, an imidazole fungicide used in agriculture and in veterinary and human medicine, amplified the effects of inbreeding on key reproductive traits.[53] Embryo viability was significantly reduced in inbred exposed fish and there was a tendency for inbred males to sire fewer offspring.
In medical research
Cancer
Zebrafish have been used to make several transgenic models of cancer, including melanoma, leukemia, pancreatic cancer and hepatocellular carcinoma.[54][55] Zebrafish expressing mutated forms of either the BRAF or NRAS oncogenes develop melanoma when placed onto a p53 deficient background. Histologically, these tumors strongly resemble the human disease, are fully transplantable, and exhibit large-scale genomic alterations. The BRAF melanoma model was utilized as a platform for two screens published in March 2011 in the journal Nature. In one study, by Ceol, Houvras and Zon, the model was used as a tool to understand the functional importance of genes known to be amplified and overexpressed in human melanoma.[56] One gene, SETDB1, markedly accelerated tumor formation in the zebrafish system, demonstrating its importance as a new melanoma oncogene. This was particularly significant because SETDB1 is known to be involved in the epigenetic regulation that is increasingly appreciated to be central to tumor cell biology.
In another study, by White and Zon, an effort was made to therapeutically target the genetic program present in the tumor's origin neural crest cell using a chemical screening approach.[57] This revealed that an inhibition of the DHODH protein (by a small molecule called leflunomide) prevented development of the neural crest stem cells which ultimately give rise to melanoma via interference with the process of transcriptional elongation. Because this approach would aim to target the "identity" of the melanoma cell rather than a single genetic mutation, leflunomide may have utility in treating human melanoma.[58]
Cardiovascular disease
In cardiovascular research, the zebrafish has been used to model blood clotting, blood vessel development, heart failure, and congenital heart and kidney disease.[59]
Immune system
In programmes of research into acute inflammation, a major underpinning process in many diseases, researchers have established a zebrafish model of inflammation, and its resolution. This approach allows detailed study of the genetic controls of inflammation and the possibility of identifying potential new drugs.[60]
Infectious diseases
As the immune system is relatively conserved between zebrafish and humans, many human infectious diseases can be modeled in zebrafish.[61][62][63][64] The transparent early life stages are well suited for in vivo imaging and genetic dissection of host-pathogen interactions.[65][66][67][68] Zebrafish models for a wide range of bacterial, viral and parasitic pathogens have already been established; for example, the zebrafish model for tuberculosis provides fundamental insights into the mechanisms of pathogenesis of mycobacteria.[69][70][71][72] Furthermore, robotic technology has been developed for high-throughput antimicrobial drug screening using zebrafish infection models.[73][74]
Repairing retinal damage

Another notable characteristic of the zebrafish is that it possesses four types of cone cell, with ultraviolet-sensitive cells supplementing the red, green and blue cone cell subtypes found in humans. Zebrafish can thus observe a very wide spectrum of colours. The species is also studied to better understand the development of the retina; in particular, how the cone cells of the retina become arranged into the so-called 'cone mosaic'. Zebrafish, in addition to certain other teleost fish, are particularly noted for having extreme precision of cone cell arrangement.[75]
This study of the zebrafish's retinal characteristics has also extrapolated into medical enquiry. In 2007, researchers at University College London grew a type of zebrafish adult stem cell found in the eyes of fish and mammals that develops into neurons in the retina. These could be injected into the eye to treat diseases that damage retinal neurons—nearly every disease of the eye, including macular degeneration, glaucoma, and diabetes-related blindness. The researchers studied Müller glial cells in the eyes of humans aged from 18 months to 91 years, and were able to develop them into all types of retinal neurons. They were also able to grow them easily in the lab. The stem cells successfully migrated into diseased rats' retinas, and took on the characteristics of the surrounding neurons. The team stated that they intended to develop the same approach in humans.[76]
Drug discovery
As demonstrated through ongoing research programmes, the zebrafish model enables researchers not only to identify genes that might underlie human disease, but also to develop novel therapeutic agents in drug discovery programmes.[77] Zebrafish embryos have proven to be a rapid, cost-efficient, and reliable teratology assay model.[78] Drug screens in zebrafish can be used to identify novel classes of compounds with biological effects, or to repurpose existing drugs for novel uses; an example of the latter would be a screen which found that a commonly used statin (rosuvastatin) can suppress the growth of prostate cancer [79] To date, 65 small-molecule screens have been carried out and at least one has led to clinical trials.[80] However, many technical challenges remain to be resolved, including differing rates of drug absorption and high levels of natural variation between individual animals.[80]
See also
- Danionin
- Japanese Brown Frog, which has been modified to develop translucent skin
- List of freshwater aquarium fish species
- ZebraBox, a specialised container for the scientific study of zebrafish
References
- ↑ Froese, Rainer and Pauly, Daniel, eds. (2007). "Danio rerio" in FishBase. March 2007 version.
- ↑ "Zebra Danio".
- 1 2 "In Memory of George Streisinger, "Founding Father" of Zebrafish Developmental and Genetic Research". University of Oregon. Retrieved September 23, 2015.
- 1 2 Goldshmit, Yona; Sztal, Tamar E.; Jusuf, Patricia R.; Hall, Thomas E.; Nguyen-Chi, Mai; Currie, Peter D. (2012). "Fgf-Dependent Glial Cell Bridges Facilitate Spinal Cord Regeneration in Zebrafish". The Journal of Neuroscience. 32 (22): 7477–92. doi:10.1523/JNEUROSCI.0758-12.2012. PMID 22649227. Lay summary – Sci-News.com (June 1, 2012).
- 1 2 "Fudan scientists turn fish into estrogen alerts". Xinhua. January 12, 2007. Retrieved November 15, 2012.
- 1 2 3 White, Richard Mark; Sessa, Anna; Burke, Christopher; Bowman, Teresa; Leblanc, Jocelyn; Ceol, Craig; Bourque, Caitlin; Dovey, Michael; et al. (2008). "Transparent Adult Zebrafish as a Tool for in Vivo Transplantation Analysis". Cell Stem Cell. 2 (2): 183–9. doi:10.1016/j.stem.2007.11.002. PMC 2292119
 . PMID 18371439. Lay summary – LiveScience (February 6, 2008).
. PMID 18371439. Lay summary – LiveScience (February 6, 2008). - 1 2 "Researchers Capture A Zebrafish's Thought Process On Video". Popular Science. January 31, 2013. Retrieved February 4, 2013.
- 1 2 Mayden, Richard L.; Tang, Kevin L.; Conway, Kevin W.; Freyhof, Jörg; Chamberlain, Sarah; Haskins, Miranda; Schneider, Leah; Sudkamp, Mitchell; et al. (2007). "Phylogenetic relationships of Danio within the order Cypriniformes: A framework for comparative and evolutionary studies of a model species". Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 308B (5): 642–54. doi:10.1002/jez.b.21175. PMID 17554749.
- 1 2 3 4 Parichy, D M (2006). "Evolution of danio pigment pattern development". Heredity. 97 (3): 200–10. doi:10.1038/sj.hdy.6800867. PMID 16835593.
- ↑ "The Zebrafish Book". ZFIN. Retrieved July 3, 2013.
- 1 2 "Danio rerio". Nonindigenous Aquatic Species. United States Geological Survey. June 14, 2013. Retrieved July 3, 2013.
- 1 2 3 4 Spence, Rowena; Gerlach, Gabriele; Lawrence, Christian; Smith, Carl (2007). "The behaviour and ecology of the zebrafish, Danio rerio". Biological Reviews. 83 (1): 13–34. doi:10.1111/j.1469-185X.2007.00030.x. PMID 18093234.
- ↑ Gerhard, G. S.; Kauffman, E. J.; Wang, X; Stewart, R; Moore, J. L.; Kasales, C. J.; Demidenko, E; Cheng, K. C. (2002). "Life spans and senescent phenotypes in two strains of Zebrafish (Danio rerio)". Exp. Gerontol. NCBI. 37 (8–9): 1055–68. doi:10.1016/s0531-5565(02)00088-8. PMID 12213556.
- 1 2 Dockser, Amy (January 13, 2012). "Birds Do It, Bees Do It, Even Zebrafish Do It—Just Too Little". Wall Street Journal. Retrieved February 11, 2012.
- ↑ Gerhard, Glenn S.; Cheng, Keith C. (2002). "A call to fins! Zebrafish as a gerontological model". Aging Cell. 1 (2): 104–11. doi:10.1046/j.1474-9728.2002.00012.x. PMID 12882339.
- ↑ Watanabe, Masakatsu; Iwashita, Motoko; Ishii, Masaru; Kurachi, Yoshihisa; Kawakami, Atsushi; Kondo, Shigeru; Okada, Norihiro (2006). "Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene". EMBO Reports. 7 (9): 893–7. doi:10.1038/sj.embor.7400757. PMC 1559663
 . PMID 16845369.
. PMID 16845369. - ↑ Mills, Dick (1993). Eyewitness Handbook: Aquarium Fish. Harper Collins. ISBN 0-7322-5012-9.
- ↑ CZRC website
- ↑ "ZFIN". ZFIN. Retrieved July 22, 2012.
- ↑ Xiang, Jing; Yang, Hongbo; Che, Chao; Zou, Haixia; Yang, Hanshuo; Wei, Yuquan; Quan, Junmin; Zhang, Hui; et al. (2009). Isalan, Mark, ed. "Identifying Tumor Cell Growth Inhibitors by Combinatorial Chemistry and Zebrafish Assays". PLoS ONE. 4 (2): e4361. Bibcode:2009PLoSO...4.4361X. doi:10.1371/journal.pone.0004361. PMC 2633036
 . PMID 19194508.
. PMID 19194508. - ↑ Hill, A. J.; Teraoka, H; Heideman, W; Peterson, RE (2005). "Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity". Toxicological Sciences. 86 (1): 6–19. doi:10.1093/toxsci/kfi110. PMID 15703261.
- ↑ Bugel, S.M.; Tanguay, R.L.; Planchart, A (2015). "Zebrafish: A marvel of high-throughput biology for 21(st) century toxicology". Current Environmental Health Reports. 1 (4): 341–352. doi:10.1007/s40572-014-0029-5. PMC 4321749
 . PMID 25678986.
. PMID 25678986. - ↑ Major, Robert J.; Poss, Kenneth D. (2007). "Zebrafish heart regeneration as a model for cardiac tissue repair". Drug Discovery Today: Disease Models. 4 (4): 219–25. doi:10.1016/j.ddmod.2007.09.002. PMC 2597874
 . PMID 19081827.
. PMID 19081827. - ↑ "Adult Stem Cell Research Avoids Ethical Concerns". Voice of America. 19 May 2010. Retrieved 21 June 2013.
- ↑ Dahm, Ralf (2006). "The Zebrafish Exposed". American Scientist. 94 (5): 446–53. doi:10.1511/2006.61.446.
- ↑ Jones, Rachel (2007). "Let Sleeping Zebrafish Lie: A New Model for Sleep Studies". PLoS Biology. 5 (10): e281. doi:10.1371/journal.pbio.0050281. PMC 2020498
 . PMID 20076649.
. PMID 20076649. - ↑ Penglase, Sam; Moren, Mari; Hamre, Kristin (2012). "Lab animals: Standardize the diet for zebrafish model". Nature: Correspondence. 491 (7424): 333. Bibcode:2012Natur.491..333P. doi:10.1038/491333a.
- ↑ Jurynec, Michael J.; Xia, Ruohong; Mackrill, John J.; Gunther, Derrick; Crawford, Thomas; Flanigan, Kevin M.; Abramson, Jonathan J.; Howard, Michael T.; Grunwald, David Jonah (2008-08-26). "Selenoprotein N is required for ryanodine receptor calcium release channel activity in human and zebrafish muscle". Proceedings of the National Academy of Sciences of the United States of America. 105 (34): 12485–12490. Bibcode:2008PNAS..10512485J. doi:10.1073/pnas.0806015105. ISSN 1091-6490. PMC 2527938
 . PMID 18713863.
. PMID 18713863. - ↑ Rederstorff, Mathieu; Castets, Perrine; Arbogast, Sandrine; Lainé, Jeanne; Vassilopoulos, Stéphane; Beuvin, Maud; Dubourg, Odile; Vignaud, Alban; Ferry, Arnaud; Krol, Alain; Allamand, Valérie; Guicheney, Pascale; Ferreiro, Ana; Lescure, Alain (2011). "Increased Muscle Stress-Sensitivity Induced by Selenoprotein N Inactivation in Mouse: A Mammalian Model for SEPN1-Related Myopathy". PLoS ONE. 6 (8): e23094. Bibcode:2011PLoSO...623094R. doi:10.1371/journal.pone.0023094. PMC 3152547
 . PMID 21858002.
. PMID 21858002. - ↑ Wade, Nicholas (March 24, 2010). "Research Offers Clue Into How Hearts Can Regenerate in Some Species". The New York Times.
- 1 2 Lush, Mark E.; Piotrowski, Tatjana (2013). "Sensory hair cell regeneration in the zebrafish lateral line". Developmental Dynamics. 243 (10): 1187–1202. doi:10.1002/dvdy.24167. PMID 25045019.
- ↑ "Mending Broken Hearts (2011) British Heart Foundation TV ad". British Heart Foundation via YouTube. January 31, 2011. Retrieved November 15, 2012.
- ↑ "British Heart Foundation – The science behind the appeal". Bhf.org.uk. February 16, 2007. Archived from the original on 10 March 2012. Retrieved November 15, 2012.
- ↑ Bernardos, Rebecca L.; Barthel, Linda K.; Meyers, Jason R.; Raymond, Pamela A. (2007). "Late-Stage Neuronal Progenitors in the Retina Are Radial Muller Glia That Function as Retinal Stem Cells". Journal of Neuroscience. 27 (26): 7028–40. doi:10.1523/JNEUROSCI.1624-07.2007. PMID 17596452.
- ↑ Stewart, Scott; Tsun, Zhi-Yang; Izpisua Belmonte, Juan Carlos (2009). "A histone demethylase is necessary for regeneration in zebrafish". Proceedings of the National Academy of Sciences. 106 (47): 19889–94. doi:10.1073/pnas.0904132106. JSTOR 25593294. PMC 2785262
 . PMID 19897725. Lay summary – Science Daily (November 2, 2009).
. PMID 19897725. Lay summary – Science Daily (November 2, 2009). - 1 2 Head, J.R.; Gacioch, L.; Pennisi; Meyers, J.R. (2013). "Activation of canonical Wnt/B-catenin signaling stimulates proliferation in neuromasts in the zebrafish posterior lateral line". Developmental Dynamics. 242 (7): 832–846. doi:10.1002/dvdy.23973. PMID 23606225.
- ↑ Steiner, A.B.; et al. (2014). "Dynamic gene expression by putative hair-cell progenitors during regeneration in the zebrafish lateral line". Proceedings of the National Academy of Sciences of the United States of America. 111 (14): 1392–1401. Bibcode:2014PNAS..111E1393S. doi:10.1073/pnas.1318692111.
- ↑ "The zebrafish as a model for muscular dystrophy and congenital myopathy". Human Molecular Genetics. August 8, 2003. Retrieved March 6, 2013.
- ↑ Kimmel, Charles B.; Law, Robert D. (1985). "Cell lineage of zebrafish blastomeres". Developmental Biology. 108 (1): 78–85. doi:10.1016/0012-1606(85)90010-7. PMID 3972182.
- ↑ Kimmel, Charles B.; Law, Robert D. (1985). "Cell lineage of zebrafish blastomeres". Developmental Biology. 108 (1): 94–101. doi:10.1016/0012-1606(85)90012-0. PMID 3972184.
- ↑ "In Vivo Testing of MicroRNA-Mediated Gene Knockdown in Zebrafish". Journal of Biomedicine and Biotechnology. Hindawi. 2012. Retrieved July 3, 2013.
- ↑ Tan, P. K.; Downey, T. J.; Spitznagel Jr, E. L.; Xu, P; Fu, D; Dimitrov, D. S.; Lempicki, R. A.; Raaka, B. M.; Cam, M. C. (2003). "Evaluation of gene expression measurements from commercial microarray platforms". Nucleic Acids Res. NCBI. 31 (19): 5676–84. doi:10.1093/nar/gkg763. PMC 206463
 . PMID 14500831.
. PMID 14500831. - ↑ "Genome Reference Consortium". GRC. Retrieved October 23, 2012.
- ↑ "Decoding the Genome Mystery". Indian Express. July 5, 2009. Retrieved February 5, 2013.
- ↑ FishMap Zv8. Institute of Genomics and Integrative Biology (IGIB). Retrieved June 7, 2012.
- ↑ Howe, Kerstin; et al. (2013). "The zebrafish reference genome sequence and its relationship to the human genome". Nature. 496: 498–503. doi:10.1038/nature12111.
- 1 2 Broughton, Richard E.; Milam, Jami E.; Roe, Bruce A. (2001). "The Complete Sequence of the Zebrafish (Danio rerio) Mitochondrial Genome and Evolutionary Patterns in Vertebrate Mitochondrial DNA". Genome Research. 11 (11): 1958–67. doi:10.1101/gr.156801 (inactive 2016-06-17). PMC 311132
 . PMID 11691861.
. PMID 11691861. - ↑ Lister, J.A.; Robertson, C.P.; Lepage, T.; Johnson, S.L.; Raible, D.W. (Sep 1999). "nacre encodes a zebrafish microphthalmia-related protein that regulates neural-crest-derived pigment cell fate". Development. 126 (17): 3757–3767. PMID 10433906.
- ↑ Lamason, R. L.; Mohideen, MA; Mest, JR; Wong, AC; Norton, HL; Aros, MC; Jurynec, MJ; Mao, X; et al. (2005). "SLC24A5, a Putative Cation Exchanger, Affects Pigmentation in Zebrafish and Humans". Science. 310 (5755): 1782–6. Bibcode:2005Sci...310.1782L. doi:10.1126/science.1116238. PMID 16357253.
- ↑ Kawakami, Koichi; Takeda, Hisashi; Kawakami, Noriko; Kobayashi, Makoto; Matsuda, Naoto; Mishina, Masayoshi (2004). "A Transposon-Mediated Gene Trap Approach Identifies Developmentally Regulated Genes in Zebrafish". Developmental Cell. 7 (1): 133–44. doi:10.1016/j.devcel.2004.06.005. PMID 15239961.
- ↑ Lin, Chien-Ling; Taggart, Allison J.; Lim, Kian Huat; Cygan, Kamil J.; Ferraris, Luciana; Creton, Robert; Huang, Yen-Tsung; Fairbrother, William G. (13 November 2015). "RNA structure replaces the need for U2AF2 in splicing". Genome Research. 26 (1): 12–23. doi:10.1101/gr.181008.114. PMC 4691745
 . PMID 26566657.
. PMID 26566657. - ↑ Charlesworth D, Willis JH (2009). "The genetics of inbreeding depression". Nat. Rev. Genet. 10 (11): 783–96. doi:10.1038/nrg2664. PMID 19834483.
- ↑ Bickley LK, Brown AR, Hosken DJ, Hamilton PB, Le Page G, Paull GC, Owen SF, Tyler CR (2013). "Interactive effects of inbreeding and endocrine disruption on reproduction in a model laboratory fish". Evol Appl. 6 (2): 279–89. doi:10.1111/j.1752-4571.2012.00288.x. PMC 3689353
 . PMID 23798977.
. PMID 23798977. - ↑ Liu, S; Leach, S. D. (2011). "Zebrafish models for cancer". Annu. Rev. Pathol. 6: 71–93. doi:10.1146/annurev-pathol-011110-130330. PMID 21261518.
- ↑ "Zebrafish model of human melanoma reveals new cancer gene". Science Daily. March 23, 2011. Retrieved April 28, 2014.
- ↑ Ceol, Craig J.; Houvras, Yariv; Jane-Valbuena, Judit; Bilodeau, Steve; Orlando, David A.; Battisti, Valentine; Fritsch, Lauriane; Lin, William M.; et al. (2011). "The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset". Nature. 471 (7339): 513–7. Bibcode:2011Natur.471..513C. doi:10.1038/nature09806. PMC 3348545
 . PMID 21430779.
. PMID 21430779. - ↑ White, Richard Mark; Cech, Jennifer; Ratanasirintrawoot, Sutheera; Lin, Charles Y.; Rahl, Peter B.; Burke, Christopher J.; Langdon, Erin; Tomlinson, Matthew L.; et al. (2011). "DHODH modulates transcriptional elongation in the neural crest and melanoma". Nature. 471 (7339): 518–22. Bibcode:2011Natur.471..518W. doi:10.1038/nature09882. PMC 3759979
 . PMID 21430780.
. PMID 21430780. - ↑ "Arthritis Drug Could Help Beat Melanoma Skin Cancer, Study Finds". Science Daily. March 24, 2011. Retrieved November 15, 2012.
- ↑ Drummond, I. A. (2005). "Kidney development and disease in the zebrafish". J. Am. Soc. Nephrol. NCBI. 16 (2): 299–304. doi:10.1681/ASN.2004090754. PMID 15647335.
- ↑ "Investigating inflammatory disease using zebrafish". Fish For Science. Retrieved November 15, 2012.
- ↑ Meeker, Nathan D.; Trede Nikolaus, S. (2008). "Immunology and zebrafish: spawning new models of human disease". Dev Comp Immunol. 32 (7): 745–757. doi:10.1016/j.dci.2007.11.011. PMID 18222541.
- ↑ Renshaw, S.A.; Trede, N.S. (2012). "A model 450 million years in the making: zebrafish and vertebrate immunity". Dis Model Mech. 5 (1): 38–47. doi:10.1242/dmm.007138. PMC 3255542
 . PMID 22228790.
. PMID 22228790. - ↑ Meijer, A.H.; Spaink, H.P. (2011). "Host–pathogen interactions made transparent with the zebrafish model". Curr Drug Targets. 12 (7): 1000–1017. doi:10.2174/138945011795677809. PMC 3319919
 . PMID 21366518.
. PMID 21366518. - ↑ Van der Vaart, M; Spaink, HP; Meijer, AH (2012). "Pathogen recognition and activation of the innate immune response in zebra fish". Adv Hematol. 2012: 159807. doi:10.1155/2012/159807. PMC 3395205
 . PMID 22811714.
. PMID 22811714. - ↑ Benard, EL; Van Der Sar, AM; Ellett, F; Lieschke, GJ; Spaink, HP; Meijer, AH (2012). "Infection of zebrafish embryos with intracellular bacterial pathogens". J Vis Exp. (61). doi:10.3791/3781. PMC 3415172
 . PMID 22453760.
. PMID 22453760. - ↑ Meijer, AH; van der Vaart, M; Spaink, HP (2013). "Real-time imaging and genetic dissection of host-microbe interactions in zebrafish". Cell Microbiol. 16: 39–49. doi:10.1111/cmi.12236. PMID 24188444.
- ↑ Torraca, V; Masud, S; Spaink, HP; Meijer, AH (Jul 2014). "Macrophage-pathogen interactions in infectious diseases: new therapeutic insights from the zebrafish host model". Dis Model Mech. 7 (7): 785–97. doi:10.1242/dmm.015594. PMC 4073269
 . PMID 24973749.
. PMID 24973749. - ↑ Levraud, JP; Palha, N; Langevin, C; Boudinot, P (Sep 2014). "Through the looking glass: witnessing host-virus interplay in zebrafish". Trends Microbiol. 22 (9): 490–7. doi:10.1016/j.tim.2014.04.014. PMID 24865811.
- ↑ Ramakrishnan, L (2013). "Looking within the zebrafish to understand the tuberculous granuloma". Adv Exp Med Biol. Advances in Experimental Medicine and Biology. 783: 251–66. doi:10.1007/978-1-4614-6111-1_13. ISBN 978-1-4614-6110-4. PMID 23468113.
- ↑ Ramakrishnan, L (2013). "The zebrafish guide to tuberculosis immunity and treatment". Cold Spring Harb Symp Quant Biol. 78: 179–92. doi:10.1101/sqb.2013.78.023283. PMID 24643219.
- ↑ Cronan, MR; Tobin, DM (Jul 2014). "Fit for consumption: zebrafish as a model for tuberculosis". Dis Model Mech. 7 (7): 777–84. doi:10.1242/dmm.016089. PMC 4073268
 . PMID 24973748.
. PMID 24973748. - ↑ Meijer, AH (2015). "Protection and pathology in TB: learning from the zebrafish model". Semin Immunopathol. doi:10.1007/s00281-015-0522-4. PMID 26324465.
- ↑ Spaink, HP; Cui, C; Wiweger, MI; Jansen, HJ; Veneman, WJ; Marín-Juez, R; de Sonneville, J; Ordas, A; Torraca, V; van der Ent, W; Leenders, WP; Meijer, AH; Snaar-Jagalska, BE; Dirks, RP (Aug 2013). "Robotic injection of zebrafish embryos for high-throughput screening in disease models". Methods. 62 (3): 246–54. doi:10.1016/j.ymeth.2013.06.002. PMID 23769806.
- ↑ Veneman, WJ; Marín-Juez, R; de Sonneville, J; Ordas, A; Jong-Raadsen, S; Meijer, AH; Spaink, HP (Jun 2014). "Establishment and optimization of a high throughput setup to study Staphylococcus epidermidis and Mycobacterium marinum infection as a model for drug discovery". J Vis Exp. 88: e51649. doi:10.3791/51649. PMC 4206090
 . PMID 24998295.
. PMID 24998295. - ↑ Allison, W. Ted; Barthel, Linda K.; Skebo, Kristina M.; Takechi, Masaki; Kawamura, Shoji; Raymond, Pamela A. (2010). "Ontogeny of cone photoreceptor mosaics in zebrafish". The Journal of Comparative Neurology. 518 (20): 4182–95. doi:10.1002/cne.22447. PMC 3376642
 . PMID 20878782.
. PMID 20878782. - ↑ Lawrence, Jean M.; Singhal, Shweta; Bhatia, Bhairavi; Keegan, David J.; Reh, Thomas A.; Luthert, Philip J.; Khaw, Peng T.; Limb, Gloria Astrid (2007). "MIO-M1 Cells and Similar Müller Glial Cell Lines Derived from Adult Human Retina Exhibit Neural Stem Cell Characteristics". Stem Cells. 25 (8): 2033–43. doi:10.1634/stemcells.2006-0724. PMID 17525239. Lay summary – The China Post (August 3, 2007).
- ↑ "Fish for Science". University of Sheffield. 2011. Retrieved March 19, 2011.
- ↑ Brannen, Kimberly C.; Panzica-Kelly, Julieta M.; Danberry, Tracy L.; Augustine-Rauch, Karen A. (2010). "Development of a zebrafish embryo teratogenicity assay and quantitative prediction model". Birth Defects Research Part B: Developmental and Reproductive Toxicology. 89 (1): 66–77. doi:10.1002/bdrb.20223. PMID 20166227.
- ↑ Rennekamp, Andrew J; Peterson, Randall T (2015-02-01). "15 years of zebrafish chemical screening". Current Opinion in Chemical Biology. Omics. 24: 58–70. doi:10.1016/j.cbpa.2014.10.025. PMC 4339096
 . PMID 25461724.
. PMID 25461724. - 1 2 MacRae, Calum A.; Peterson, Randall T. "Zebrafish as tools for drug discovery". Nature Reviews Drug Discovery. 14 (10): 721–731. doi:10.1038/nrd4627.
Further reading
- "Danio rerio". Integrated Taxonomic Information System. Retrieved November 12, 2004.
- Lambert, Derek J (1997). Freshwater Aquarium Fish. Edison, New Jersey: Chartwell Books. p. 19. ISBN 0-7858-0867-1.
- Sharpe, Shirlie. "Zebra Danio". Your Guide to Freshwater Aquariums. Retrieved December 15, 2004.
- Kocher, Thomas D.; Jeffery, WR; Parichy, DM; Peichel, CL; Streelman, JT; Thorgaard, GH (2005). "Fish Models for Studying Adaptive Evolution and Speciation". Zebrafish. 2 (3): 147–56. doi:10.1089/zeb.2005.2.147. PMID 18248189.
- Bradbury, Jane (2004). "Small Fish, Big Science". PLoS Biology. 2 (5): e148. doi:10.1371/journal.pbio.0020148. PMC 406403
 . PMID 15138510.
. PMID 15138510. - Westerfield, M. (2007). The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) (5th ed.). Eugene, OR: University of Oregon Press.
- Guttridge, Nicky (2012). "Targeted gene modification can rewrite zebrafish DNA". Nature. doi:10.1038/nature.2012.11463.
- "A Point Of View: Fly, Fish, Mouse and Worm". BBC. June 14, 2013. Retrieved June 15, 2013.
External links
| Wikimedia Commons has media related to Danio rerio. |
- British Association of Zebrafish Husbandry
- The Zebrafish Information Network (ZFIN)
- The Zebrafish International Resource Center (ZIRC)
- The China Zebrafish Resource Center (CZRC)
- The Zebrafish Genome Sequencing Project at the Wellcome Trust Sanger Institute
- FishMap: The Zebrafish Community Genomics Browser at the Institute of Genomics and Integrative Biology (IGIB)
- WebHome Zebrafish GenomeWiki Beta Preview at the IGIB
- Genome sequencing initiative at the IGIB
- Danio rerio at Danios.info
- Sanger Institute Zebrafish Mutation Resource
- Zebrafish genome via Ensembl
- FishforScience.com – using zebrafish for medical research
- FishForPharma
- View the danRer10 genome assembly in the UCSC Genome Browser.
