Diazo

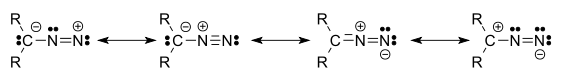
Diazo refers to a type of organic compound called diazo compound that has two linked nitrogen atoms (azo) as a terminal functional group. The general formula is R2C=N2. The simplest example of a diazo compound is diazomethane. The electronic structure of diazo compounds involves a positive charge on the central nitrogen and negative charge distributed between the terminal nitrogen and the carbon. Some of the most stable diazo compounds are α-diazo-ß-diketones and α-diazo-ß-diesters since the negative charge is delocalized into the carbonyls. In contrast, most alkyldiazo compounds are explosive. A commercially relevant diazo compound is ethyl diazoacetate (N2CHCOOEt). A group of isomeric compounds with only few similar properties are the diazirines, where the carbon and two nitrogens are linked as a ring.
Four resonance structures can be drawn:[1]
Diazo compounds should not be confused with azo compounds of the type R-N=N-R or with diazonium compounds of the type R-N2+.
History
Diazo compounds were first produced by Peter Griess who had discovered a versatile new chemical reaction, as detailed in his 1858 paper "Preliminary notice on the influence of nitrous acid on aminonitro- and aminodinitrophenol." [2][3]
Diazo synthesis
Several laboratory methods exist for the preparation of diazo compounds:[4][5]
From amines
Alpha-acceptor-substituted primary aliphatic amines R-CH2-NH2 (R = COOR, CN, CHO, COR) react with nitrous acid to generate the diazo compound.
From diazomethyl compounds
An example of an electrophilic substitution using a diazomethyl compound is that of a reaction between an acyl halide and diazomethane,[6] for example the first step in the Arndt-Eistert synthesis.
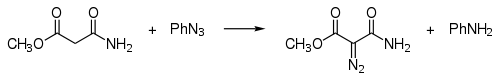
By diazo transfer
In diazo transfer certain carbon acids can be reacted with tosyl azide:
This reaction is also called the Regitz diazo transfer.[7] Examples are the synthesis of tert-butyl diazoaceate[8] and di-tert-butyl diazomalonate.[9]
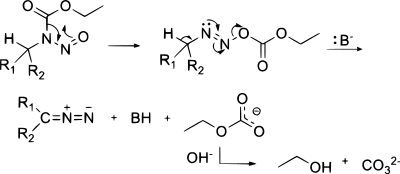
From N-alkyl-N-nitroso compounds
Diazo compounds can be obtained in an elimination reaction of N-alkyl-N-nitroso compounds,[10] such as in the synthesis of diazomethane from Diazald or MNNG:
From hydrazones
Hydrazones are oxidized (dehydrogenation) for example with silver oxide or mercury oxide for example the synthesis of 2-diazopropane from acetone hydrazone.[11] Other oxidizing reagents are lead tetraacetate, manganese dioxide and the Swern reagent. Tosylhydrazones RRC=N-NHTs are reacted with base for example triethylamine in the synthesis of crotyl diazoacetate[12] and in the synthesis of phenyldiazomethane from PhCHNHTs and sodium methoxide.[13]
Reaction of a carbonyl group with the hydrazine 1,2-bis(tert-butyldimethylsilyl)hydrazine to form the hydrazone is followed by reaction with the periodinane difluoroiodobenzene yields the diazo compound:[14][15]
By fragmentation
1,3-disubstituted alkyl aryl triazenes can be fragmentated to form diazo compounds. These triazenes (ArN=NNH-CH2R) result from coupling of aromatic diazonium salts with primary amines but the reaction type is rare.
From azides
One method is described for the synthesis of diazo compounds from azides using phosphines:[16]
Diazo reactions
In cycloadditions
Diazo compounds react as 1,3-dipoles in diazoalkane 1,3-dipolar cycloadditions.
As carbene precursors
Diazo compounds are used as precursors to carbenes, which are generated by thermolysis or photolysis, for example in the Wolff rearrangement. As such they are used in cyclopropanation for example in the reaction of ethyl diazoacetate with styrene.[17] Certain diazo compounds can couple to form alkenes in a formal carbene dimerization reaction.
Diazo compounds are intermediates in the Bamford-Stevens reaction of tosylhydrazones to alkenes, again with a carbene intermediate:
In the Doyle-Kirmse reaction certain diazo compounds react with allyl sulfides to the homoallyl sulfide. Intramolecular reactions of diazocarbonyl compounds provide access to cyclopropanes. In the Buchner ring expansion diazo compounds react with aromatic rings with ring-expansion.
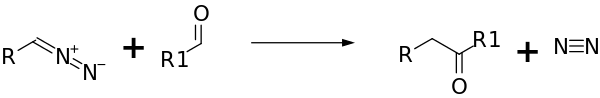
As nucleophile
The Buchner-Curtius-Schlotterbeck Reaction yields ketones from aldehydes and aliphatic diazo compounds:
The reaction type is nucleophilic addition.
Occurrence in nature
Two families of naturally occurring products feature the diazo group: kinamycin and lomaiviticin. These molecules are DNA-intercalators, with diazo functionality as their "warheads". Loss of N2, induced reductively, generates a DNA-cleaving fluorenyl radical.
See also
References
- ↑ F.A. Carey R.J. Sundberg Advanced Organic Chemistry, 2nd Edition
- ↑ Trevor I. Williams, ‘Griess, (Johann) Peter (1829–1888)’, Oxford Dictionary of National Biography, Oxford University Press, 2004
- ↑ Peter Griess (1858) "Vorläufige Notiz über die Einwirkung von salpetriger Säure auf Amidinitro- und Aminitrophenylsäure," (Preliminary notice of the reaction of nitrous acid with picramic acid and aminonitrophenol), Annalen der Chemie und Pharmacie, 106 : 123-125.
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ↑ New Syntheses of Diazo Compounds Gerhard Maas Angew. Chem. Int. Ed. 2009, 48, 8186 – 8195 doi:10.1002/anie.200902785
- ↑ Example Organic Syntheses, Coll. Vol. 3, p.119 (1955); Vol. 26, p.13 (1946).Link
- ↑ M. Regitz, Angew. Chem., 79, 786 (1967); Angew. Chem. Intern. Ed. Engl., 6, 733 (1967).
- ↑ Organic Syntheses, Coll. Vol. 5, p.179 (1973); Vol. 48, p.36 (1968). Link
- ↑ Organic Syntheses, Coll. Vol. 6, p.414 (1988); Vol. 59, p.66 (1979). Link
- ↑ Example: Organic Syntheses, Coll. Vol. 6, p.981 (1988); Vol. 57, p.95 (1977). Link
- ↑ Organic Syntheses, Coll. Vol. 6, p.392 (1988); Vol. 50, p.27 (1970). Link
- ↑ Organic Syntheses, Coll. Vol. 5, p.258 (1973); Vol. 49, p.22 (1969). Link
- ↑ Organic Syntheses, Coll. Vol. 7, p.438 (1990); Vol. 64, p.207 (1986).http://www.orgsyn.org/orgsyn/prep.asp?prep=CV7P0438
- ↑ Lei, X.; Porco Ja, J. (2006). "Total synthesis of the diazobenzofluorene antibiotic (-)-kinamycin C1". Journal of the American Chemical Society. 128 (46): 14790–14791. doi:10.1021/ja066621v. PMID 17105273.
- ↑ Elusive Natural Product Is Synthesized Stu Borman Chemical & Engineering News October 31, 2006 Link.
- ↑ A Phosphine-Mediated Conversion of Azides into Diazo Compounds Eddie L. Myers and Ronald T. Raines Angew. Chem. Int. Ed. 2009, 48, 2359 –2363 doi:10.1002/anie.200804689
- ↑ Organic Syntheses, Coll. Vol. 6, p.913 (1988); Vol. 50, p.94 (1970).Link