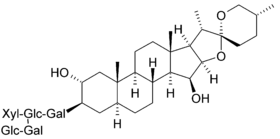
Digitonin
 | |
| Names | |
|---|---|
| Other names
digitin | |
| Identifiers | |
| 11024-24-1 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL404811 |
| ChemSpider | 23753 |
| ECHA InfoCard | 100.031.129 |
| EC Number | 234-255-6 |
| KEGG | C00765 |
| PubChem | 25444 |
| |
| |
| Properties | |
| C56H92O29 | |
| Molar mass | 1229.3 g/mol |
| Melting point | 244.0–248.5 °C (471.2–479.3 °F; 517.1–521.6 K)[1] |
| Chiral rotation ([α]D) |
-40 ° (Wavlen: 589.3 nm; Temp: 20 °C)[1] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
23 mg/kg (rat, intravenous)[2]
4 mg/kg (mouse, intravenous)[3] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Digitonin is a steroidal saponin (saraponin) obtained from the foxglove plant Digitalis purpurea. Its aglycone is digitogenin, a spirostan steroid. It has been investigated as a detergent, as it effectively water-solubilizes lipids. As such, it has several potential membrane-related applications in biochemistry, including solubilizing membrane proteins, precipitating cholesterol, and permeabilizing cell membranes.[4][5]
Digitonin is sometimes confused with the cardiac drug digoxin (sometimes also called digitalis or digitoxin) and both can be extracted from the same source.
Physical, chemical and biological properties
Critical micelle concentration = < 0.5 mM
Average micellar weight = 70000
Appearance = White to off-white powder
Boiling Point/Melting Point = 230 to 240 °C
Aggregation number = 60
Rat-LD50 = 4 mg/kg body weight (IVN), 51 mg/kg body weight (ORAL)
References
- 1 2 Tschesche, R.; Wulff, G. (January 1963). "Über saponine der spirostanolreihe—IX". Tetrahedron (in German). 19 (4): 621–634. doi:10.1016/S0040-4020(01)98548-5.
- ↑ Segal, Ruth; Milo-Goldzweig, Ilana; Kaplan, Gideon; Weisenberg, Emil (April 1977). "The protective action of glycyrrhizin against saponin toxicity". Biochemical Pharmacology. 26 (7): 643–645. doi:10.1016/0006-2952(77)90039-9.
- ↑ Pitha, Josef; Szente, Lajos (February 1984). "Digitonin derivatives of low toxicity: Potential solubilizers for lipophilic compounds". Journal of Pharmaceutical Sciences. 73 (2): 240–243. doi:10.1002/jps.2600730224.
- ↑ Geelen, Math J.H. (December 2005). "The use of digitonin-permeabilized mammalian cells for measuring enzyme activities in the course of studies on lipid metabolism". Analytical Biochemistry. 347 (1): 1–9. doi:10.1016/j.ab.2005.03.032.
- ↑ Fiskum, Gary (April 1985). "Intracellular levels and distribution of Ca2+ in digitonin-permeabilized cells". Cell Calcium. 6 (1-2): 25–37. doi:10.1016/0143-4160(85)90032-6.