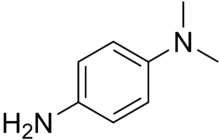
Dimethyl-4-phenylenediamine
 | |
| Names | |
|---|---|
| IUPAC name
N,N-Dimethylbenzene-1,4-diamine | |
| Other names
p-Aminodimethylaniline; N,N-Dimethyl-p-phenylenediamine; 4-(Dimethylamino)aniline; p-Amino-N,N-dimethylaniline; p-(Dimethylamino)aniline; DMPPDA; Dimethyl-p-phenylenediamine; 4-Amino-N,N-dimethylaniline; p-Dimethylaminophenylamine; DMPD | |
| Identifiers | |
| 99-98-9 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | [http://www.chemspider.com/Chemical-Structure.13884246
|
| ECHA InfoCard | 100.002.552 |
| PubChem | 7472 |
| UNII | 7GZH2FMK7X |
| |
| Properties | |
| C8H12N2 | |
| Molar mass | 136.20 g·mol−1 |
| Appearance | Reddish-violet crystals[1] |
| Melting point | 53 °C (127 °F; 326 K)[1] |
| Boiling point | 262 °C (504 °F; 535 K)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Uses
Dimethyl-4-phenylenediamine is a molecule used in the oxidase test, and an important precursor to Methylene blue.
Synthesis
In 1877 BASF was producing this compound by nitrosylation and reductive hydrogenation of dimethylaniline.
- (CH3)2N-C6H5 + HNO2 → (CH3)2N-C6H5-NO
- (CH3)2N-C6H5-NO + 2H2 → (CH3)2N-C6H5-NH2 + H2O
References
- 1 2 3 Merck Index, 11th Edition, 3242
See also http://www.sensafe.com/blog/2008/07/what-does-dpd-stand-for/
This article is issued from Wikipedia - version of the 7/1/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.