Dulcin
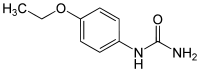 | |
| Names | |
|---|---|
| IUPAC name
(4-Ethoxyphenyl)urea | |
| Other names
Sucrol; Valzin; Dulcine; Suesstoff | |
| Identifiers | |
| 150-69-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 8663 |
| ECHA InfoCard | 100.005.244 |
| EC Number | 205-767-7 |
| KEGG | C19415 |
| PubChem | 9013 |
| RTECS number | YT2275000 |
| UNII | 8U78KF577Z |
| |
| |
| Properties | |
| C9H12N2O2 | |
| Molar mass | 180.1 g/mol |
| Appearance | white needles |
| Melting point | 173.5 °C (344.3 °F; 446.6 K) |
| Boiling point | decomp |
| 12.5 g/cm3 (25 °C) | |
| Solubility | soluble in alcohol |
| log P | 1.28 |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
1900 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dulcin is an artificial sweetener about 250 times sweeter than sugar discovered in 1884 by Joseph Berlinerbau.[1] It was first mass-produced about seven years later. Despite the fact that it was discovered only five years after saccharin, it never enjoyed the latter compound’s market success. Still, it was an important sweetener of the early 20th century and had an advantage over saccharin in that it did not possess a bitter aftertaste. It is not known to occur as a natural product.
Early medical tests marked the substance as safe for human consumption, and it was considered ideal for diabetics. However, an FDA study in 1951 raised many questions about its safety resulting in its removal from the market in 1954 after animal testing revealed unspecified carcinogenic properties. In Japan, poisoning accidents by dulcin occurred frequently, and use of dulcin was forbidden in 1969.[2]
Dulcin is also known by the names sucrol and valzin.[3]
Preparation
Dulcin is produced from the addition of potassium cyanate to p-phenetidine hydrochloride in an aqueous solution at room temperature.
An alternate way to make dulcin is by mixing urea and p-phenetidine hydrochloride to a mixture of hydrochloric acid and glacial acetic acid.
References
- ↑ Goldsmith, R.H. (1987). "A tale of two sweeteners". J. Chem. Educ. 64 (11): 954–955. doi:10.1021/ed064p954.
- ↑ ズルチン標準品-Dulcin Standard (Japanese), Wako Pure Chemical Industries
- ↑ Bender, David A. (2005). A Dictionary of Food and Nutrition. Oxford University Press.
Additional reading
- Hodges, L. 1973. Environmental pollution: a survey emphasizing physical and chemical principles. Holt, Rinehart and Winston Inc., New York.