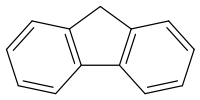
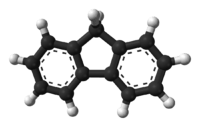
Fluorene
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
9H-Fluorene | |
| Systematic IUPAC name
Tricyclo[7.4.0.02,7]trideca-2,4,6,9,11,13-hexaene | |
| Identifiers | |
| 86-73-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:28266 |
| ChEMBL | ChEMBL16236 |
| ChemSpider | 6592 |
| ECHA InfoCard | 100.001.541 |
| EC Number | 201-695-5 |
| KEGG | C07715 |
| PubChem | 6853 |
| RTECS number | LL5670000 |
| UNII | 3Q2UY0968A |
| |
| |
| Properties | |
| C13H10 | |
| Molar mass | 166.22 g·mol−1 |
| Density | 1.202 g/mL |
| Melting point | 116 to 117 °C (241 to 243 °F; 389 to 390 K) |
| Boiling point | 295 °C (563 °F; 568 K) |
| 1.992 mg/L | |
| Solubility | soluble in CS2, ether, benzene, hot alcohol, pyrimidine, CCl4, toluene, acetone, DMSO |
| log P | 4.18 |
| Acidity (pKa) | 22.6 |
| Hazards | |
| Safety data sheet | Sigma-Aldrich |
| NFPA 704 | |
| Flash point | 152 °C (306 °F; 425 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
16000 mg/kg (oral, rat) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Fluorene /ˈflʊəriːn/, or 9H-fluorene, is a polycyclic aromatic hydrocarbon. It forms white crystals that exhibit a characteristic, aromatic odor similar to that of naphthalene. It is combustible. It has a violet fluorescence, hence its name. For commercial purposes it is obtained from coal tar.[2] It is insoluble in water and soluble in many organic solvents.
Synthesis, structure, and reactivity
Although fluorene is obtained from coal tar, it can also be prepared by dehydrogenation of diphenylmethane.[2] Alternatively, it can be prepared by the reduction of fluorenone with zinc.[3] The fluorene molecule is nearly planar,[4] although each of the two benzene rings is coplanar with the central carbon 9.[5]
Acidity
The C9-H sites of the fluorene ring are weakly acidic (pKa = 22.6 in DMSO.[6]) Deprotonation gives the stable fluorenyl "anion", nominally C13H9−, which is aromatic and has an intense orange colour. The anion is a nucleophile, and most electrophiles react with it by adding to the 9-position. The purification of fluorene exploits its acidity and the low solubility of its sodium derivative in hydrocarbon solvents.
Both protons can be removed from C9. For example, 9,9-fluorenyldipotassium can be obtained by treating fluorene with potassium metal in boiling dioxane.[7]
Uses
Fluorene is a precursor to other fluorene compounds; the parent species has few applications. Fluorene-9-carboxylic acid is a precursor to pharmaceuticals. 2-Aminofluorene, 3,6-bis-(dimethylamino)fluorene, and 2,7-diiodofluorene are precursors to dyes. Oxidation of fluorene gives fluorenone, which is nitrated to give commercially useful derivatives. 9-Fluorenylmethyl chloroformate (Fmoc chloride) is used to introduce the 9-fluorenylmethyl carbamate (Fmoc) protecting group on amines in peptide synthesis.[2]
Polyfluorene polymers (where carbon 7 of one unit is linked to carbon 2 of the next one, displacing two hydrogens) are electrically conductive and electroluminescent, and have been much investigated as a luminophore in organic light-emitting diodes.
Cicloprofen is a NSAID 2-arylpropionic acid made from fluorene.
See also
References
- ↑ Merck Index, 11th Edition, 4081
- 1 2 3 Karl Griesbaum, Arno Behr, Dieter Biedenkapp, Heinz-Werner Voges, Dorothea Garbe, Christian Paetz, Gerd Collin, Dieter Mayer, Hartmut Höke "Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry 2002 Wiley-VCH, Weinheim. doi:10.1002/14356007.a13_227
- ↑ Fittig, Rud. (1873), "Ueber einen neuen Kohlenwasserstoff aus dem Diphenylenketon" Ber. Dtsch. Chem. Ges. volume 6, p. 187.doi:10.1002/cber.18730060169
- ↑ D. M. Burns, John Iball (1954), Molecular Structure of Fluorene Nature volume 173, p. 635. doi:10.1038/173635a0
- ↑ R. E. Gerkin, A. P. Lundstedt and W. J. Reppart (1984) Structure of fluorene, C13H10, at 159 K Acta Crystallographica, volume C40, pp. 1892–1894 doi:10.1107/S0108270184009963
- ↑ F. G. Bordwell (1988). "Equilibrium acidities in dimethyl sulfoxide solution". Acc. Chem. Res. 21 (12): 456–463. doi:10.1021/ar00156a004.
- ↑ G. W. Scherf and R. K. Brown (1960), POTASSIUM DERIVATIVES OF FLUORENE AS INTERMEDIATES IN THE PREPARATION OF C9-SUBSTITUTED FLUORENES. I. THE PREPARATION OF 9-FLUORENYL POTASSIUM AND THE INFRARED SPECTRA OF FLUORENE AND SOME C9-SUBSTITUTED FLUORENES. Canadian Journal of Chemistry, Vol. 38, p. 697.
External links
- National Pollutant Inventory - Polycyclic Aromatic Hydrocarbon Fact Sheet
- Fluorene in the National Institute of Standards and Technology database.
