Free fatty acid receptor 1
| View/Edit Human | View/Edit Mouse |
Free fatty acid receptor 1 (FFA1), also known as GPR40, is a class A G-protein coupled receptor that in humans is encoded by the FFAR1 gene.[4] It is strongly expressed in the cells of the pancreas and to a lesser extent in the brain.[5] This membrane protein binds free fatty acids, acting as a nutrient sensor for regulating energy homeostasis.[6]
Activation/Inhibition
The protein FFA1 is activated by medium to long chain fatty acids. FFA1 is most strongly activated by eicosatrienoic acid (20:3Δ11,14,17), but has been found to be activated by fatty acids as small as 10 carbons long. For saturated fatty acids the level of activation is dependent on the length of the carbon chain, which is not true for unsaturated fatty acids.[6] It has been found that three hydrophilic residues (arginine-183, asparagine-244, and arginine-258) anchor the carboxylate group of a fatty acid, which activates FFA1.[7]
In the pancreas
FFA1 is found in highest concentration in pancreatic Islets of Langerhans, the endocrine portion of the pancreas.[8] Activation of FFA1 results in an increase in cytosolic Ca2+ via the phosphoinositide pathway.[6] When a free fatty acid docks on FFA1, the membrane protein becomes activated. This activation causes one of its subunits to dissociate from the receptor, which then activates phospholipase C (PLC) which is found in the cell membrane. PLC in turn hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2),which is also in the membrane, to diacyl glycerol (DAG) which stays in the membrane, and inositol 1,4,5-triphosphate (IP3), which enters the cytosol. IP3 can then dock on a calcium channel in the endoplasmic reticulum which will facilitate the release of Ca2+ into the cytosol.
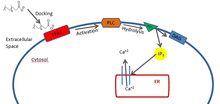
]
The Ca2+ that is released then initiates a signal cascade, resulting in the secretion of insulin.[8] A high concentration of glucose in the blood has been found to increase the transcription of the FFA1 gene, which has made these receptors a new target for the treatment of type II diabetes.[9] While fatty acids themselves do not elicit insulin secretion, FFA1 activation increases the amount of insulin being secreted through various linked pathways. It has also been shown that chronic exposure to high amounts of free fatty acids, like on a high fat diet, can impair the function and secretory capacity of pancreatic β-cells.[6]
In the brain
As stated previously, FFA1 has an affinity for long chain fatty acids. Such fatty acids are also present in the brain, where FFA1 has also been found in high abundance. FFA1 receptors are present over the entire brain, but in highest numbers in the medulla oblongata and the substantia nigra.[10] Recent studies have also observed that FFA1 was present in the olfactory bulb, striatum, hippocampus, midbrain, hypothalamus, cerebellum, cerebral cortex and in the spinal cord.[11]
Fatty acids play an important role in normal brain development as well as maintaining proper neuronal function.[12] It has been found that certain fatty acids that are in abundance in the brain may be linked with FFA1. These fatty acids likely activate FFA1, inducing an intracellular response.[12] It has been found that docosahexaenoic acid (DHA) has a higher affinity than other fatty acids for FFA1.[8] DHA makes up 30% and arachidonic acid, another fatty acid found in the brain, makes up 20% of the fatty acids in the brain.[12] Both of these fatty acids must be obtained from the diet because the body cannot make them. A correct balance of these fatty acids is vital to normal brain function and structure.[12] DHA is supplied to the brain via astrocytes, which release DHA so that it reaches a high enough concentration to act as an extracellular signal on FFA1.[12]
The abundance of FFA1 in the brain and the high affinity for DHA suggest that FFA1 may play a role in neuronal function in the brain. It is hypothesized that DHA and arachidonic acid could improve memory function by interacting with FFA1 in the hippocampus neurons.[12] This hypothesis is based on the idea that once FFA1 is activated by these fatty acids, the resulting signal is related to progenitor cell proliferation.[13] This implies that FFA1 signaling could stimulate the production of new memory cells in the brain. More research needs to be done in proving these suggestions, but if proven to be true FFA1 could be a target in producing new memory cells that are destroyed by diseases like Alzheimer's and Parkinson's disease.[10]
Additionally, FFA1 abundance in the brain has been suggested to play a role in pain. DHA has been reported to induce an increased tolerance for pain without binding to opioid receptors.[11] Researchers have hypothesized that stimulation of FFA1 by DHA could accelerate the release of endorphins, which is how DHA could induce an increased tolerance to pain.[11] DHA binds to FFA1, which could activate a signaling cascade that leads to Ca2+ influx, which then leads to accelerated endorphin release and novel pain control.[11] Again, additional research must be done to fully understand the mechanism and to prove these hypotheses, but the implications could provide additional targets for pain control in individuals.
Oral Fat Detection
FFA1(GPR40) has been implicated in the ability to taste fats.[14] It is expressed in taste bud cells (specifically cell type I), and its absence leads to reduced preference to two types of fatty acid (linoleic acid and oleic acid), as well as decreased neuronal response to fatty acids administered orally.[15]
FFA1 in Breast Cancer
FFA1 has been found to be expressed in the human breast cancer cell line MCF-7. An increase in [Ca2+], which is a consequence of FFA1 activation, has been shown to modulate processes required for breast cancer cell proliferation. This suggests that FFA1 plays a crucial role in breast cancer proliferation. To further demonstrate this connection, pertussis toxin, which is a very specific inhibitor of GPCRs like FFA1, was found to diminish cancer cell proliferation. Also, using a PLC inhibitor diminished proliferation.[16]
See also
References
- ↑ "Drugs that physically interact with Free fatty acid receptor 1 view/edit references on wikidata".
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: FFAR1 free fatty acid receptor 1".
- ↑ "UniGene: EST profile FFAR1 free fatty acid receptor 1".
- 1 2 3 4 Ichimura A, Hirasawa A, Hara T, Tsujimoto G (September 2009). "Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis". Prostaglandins Other Lipid Mediat. 89 (3-4): 82–8. doi:10.1016/j.prostaglandins.2009.05.003. PMID 19460454.
- ↑ Sum CS, Tikhonova IG, Neumann S, Engel S, Raaka BM, Costanzi S, Gershengorn MC (October 2007). "Identification of residues important for agonist recognition and activation in GPR40". J. Biol. Chem. 282 (40): 29248–55. doi:10.1074/jbc.M705077200. PMID 17699519.
- 1 2 3 Itoh Y, Kawamata Y, Harada M, Kobayashi M, Fujii R, Fukusumi S, Ogi K, Hosoya M, Tanaka Y, Uejima H, Tanaka H, Maruyama M, Satoh R, Okubo S, Kizawa H, Komatsu H, Matsumura F, Noguchi Y, Shinohara T, Hinuma S, Fujisawa Y, Fujino M (March 2003). "Free fatty acids regulate insulin secretion from pancreatic beta cells through GPR40". Nature. 422 (6928): 173–6. doi:10.1038/nature01478. PMID 12629551.
- ↑ Kebede M, Ferdaoussi M, Mancini A, Alquier T, Kulkarni RN, Walker MD, Poitout V (February 2012). "Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1". Proc. Natl. Acad. Sci. U.S.A. 109 (7): 2376–81. doi:10.1073/pnas.1114350109. PMC 3289358
 . PMID 22308370.
. PMID 22308370. - 1 2 Briscoe CP, Tadayyon M, Andrews JL, Benson WG, Chambers JK, Eilert MM, Ellis C, Elshourbagy NA, Goetz AS, Minnick DT, Murdock PR, Sauls HR, Shabon U, Spinage LD, Strum JC, Szekeres PG, Tan KB, Way JM, Ignar DM, Wilson S, Muir AI (March 2003). "The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids". J. Biol. Chem. 278 (13): 11303–11. doi:10.1074/jbc.M211495200. PMID 12496284.
- 1 2 3 4 Nakamoto K, Nishinaka T, Matsumoto K, Kasuya F, Mankura M, Koyama Y, Tokuyama S (January 2012). "Involvement of the long-chain fatty acid receptor GPR40 as a novel pain regulatory system". Brain Res. 1432: 74–83. doi:10.1016/j.brainres.2011.11.012. PMID 22137657.
- 1 2 3 4 5 6 Yamashima T (February 2008). "A putative link of PUFA, GPR40 and adult-born hippocampal neurons for memory". Prog. Neurobiol. 84 (2): 105–15. doi:10.1016/j.pneurobio.2007.11.002. PMID 18191887.
- ↑ Ma D, Lu L, Boneva NB, Warashina S, Kaplamadzhiev DB, Mori Y, Nakaya MA, Kikuchi M, Tonchev AB, Okano H, Yamashima T (2008). "Expression of free fatty acid receptor GPR40 in the neurogenic niche of adult monkey hippocampus". Hippocampus. 18 (3): 326–33. doi:10.1002/hipo.20393. PMID 18064707.
- ↑ PMID 24631296
- ↑ PMID 20573884
- ↑ Hardy, S.; St-Onge, G.G.; Joly, E.; Langelier, Y.; Prentki, M. (April 2005). "Oleate Promotes the Proliferation of Breast Cancer Cells via the G Protein-coupled Receptor GPR40". J. Biol. Sci. 280 (14): 13285–91. doi:10.1074/jbc.M410922200. PMID 15695516.
Further reading
- Brown AJ, Jupe S, Briscoe CP (2005). "A family of fatty acid binding receptors.". DNA Cell Biol. 24 (1): 54–61. doi:10.1089/dna.2005.24.54. PMID 15684720.
- Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O'Dowd BF (1997). "A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1.". Biochem. Biophys. Res. Commun. 239 (2): 543–7. doi:10.1006/bbrc.1997.7513. PMID 9344866.
- Kotarsky K, Nilsson NE, Flodgren E, Owman C, Olde B (2003). "A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs.". Biochem. Biophys. Res. Commun. 301 (2): 406–10. doi:10.1016/S0006-291X(02)03064-4. PMID 12565875.
- Hardy S, St-Onge GG, Joly E, Langelier Y, Prentki M (2005). "Oleate promotes the proliferation of breast cancer cells via the G protein-coupled receptor GPR40.". J. Biol. Chem. 280 (14): 13285–91. doi:10.1074/jbc.M410922200. PMID 15695516.
- Ogawa T, Hirose H, Miyashita K, Saito I, Saruta T (2005). "GPR40 gene Arg211His polymorphism may contribute to the variation of insulin secretory capacity in Japanese men.". Metab. Clin. Exp. 54 (3): 296–9. doi:10.1016/j.metabol.2004.09.008. PMID 15736105.
Tomita T, Masuzaki H, Noguchi M, Iwakura H, Fujikura J, Tanaka T, Ebihara K, Kawamura J, Komoto I, Kawaguchi Y, Fujimoto K, Doi R, Shimada Y, Hosoda K, Imamura M, Nakao K (2006). "GPR40 gene expression in human pancreas and insulinoma.". Biochem. Biophys. Res. Commun. 338 (4): 1788–90. doi:10.1016/j.bbrc.2005.10.161. PMID 16289108.
- Tomita T, Masuzaki H, Iwakura H, Fujikura J, Noguchi M, Tanaka T, Ebihara K, Kawamura J, Komoto I, Kawaguchi Y, Fujimoto K, Doi R, Shimada Y, Hosoda K, Imamura M, Nakao K (2006). "Expression of the gene for a membrane-bound fatty acid receptor in the pancreas and islet cell tumours in humans: evidence for GPR40 expression in pancreatic beta cells and implications for insulin secretion.". Diabetologia. 49 (5): 962–8. doi:10.1007/s00125-006-0193-8. PMID 16525841.
- Stoddart LA, Brown AJ, Milligan G (2007). "Uncovering the pharmacology of the G protein-coupled receptor GPR40: high apparent constitutive activity in guanosine 5'-O-(3-[35S]thio)triphosphate binding studies reflects binding of an endogenous agonist.". Mol. Pharmacol. 71 (4): 994–1005. doi:10.1124/mol.106.031534. PMID 17200419.
- Bartoov-Shifman R, Ridner G, Bahar K, Rubins N, Walker MD (2007). "Regulation of the gene encoding GPR40, a fatty acid receptor expressed selectively in pancreatic beta cells.". J. Biol. Chem. 282 (32): 23561–71. doi:10.1074/jbc.M702115200. PMID 17525159.