Fusicoccin
 | |
| Names | |
|---|---|
| IUPAC name
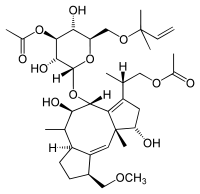
(2S)-2-[(1S,4R,5R,6R,6aS,9S,9aE,10aR)-4-{[3-O-Acetyl-6-O-(2-methyl-3-buten-2-yl)-α-D-glucopyranosyl]oxy}-1,5-dihydroxy-9-(methoxymethyl)-6,10a-dimethyl-1,2,4,5,6,6a,7,8,9,10a-decahydrodicyclopenta[a,d][8]annulen-3-yl]propyl acetate | |
| Identifiers | |
| 20108-30-9 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 394625 |
| DrugBank | DB01780 |
| PubChem | 447573 |
| |
| |
| Properties | |
| C36H56O12 | |
| Molar mass | 680.83 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fusicoccin is an organic compound produced by a fungus. It has detrimental effect on plants and causes their death.
Fusicoccin is synthesized by the fungus Fusicoccum amygdali,[1] which is a parasite of mainly almond and peach trees. It stimulates a quick acidification of the plant cell wall; this causes the stomata to irreversibly open, which brings about the death of the plant.
Fusicoccin contains three fused carbon rings and another ring which contains an oxygen atom and five carbons.
Fusicoccin was and is extensively used in research regarding the plant hormone auxin and its mechanisms.
Biosynthesis
Fusicoccin is a member of a diterpenoid class which shares a 5-8-5 ring structure and is called fusicoccane.[2] In fungi, fusicoccin is biosynthesized via Phomopsis amygdali fusicoccadiene synthase (PaFS) from universal C5 isoprene units dimethylallyl diphosphate (DMAPP) and isopentenyl diphosphate (IPP). PaFS has two domains, a C-terminal prenyltransferase domain which converts isoprene units into geranylgeranyl diphosphate (GGDP) and an N-terminal terpene cyclase domain where GGDP gets cyclized and turns into fusicocca-2,10(14)-diene. It is also reported in this study that a 2-oxoglutarate-dependent dioxygenase-like gene, a cytochrome P450 monooxygenase-like gene, a short-chain dehydrogenase/reductase-like gene, and an α-mannosidase-like gene at the 3’ location downstream of PaFS which are responsible for converting fusicocca-2,10(14)-diene into fusicoccin.[3] Two enzymes, one dioxygenase and PAPT, are in charge of catalyzing a hydroxylation at the 3-position of fusicocca-2,10(14)-diene-8β,16-diol and prenylation of the hydroxyl group of glucose in fusicoccin P, respectively.[4][5]

References
- ↑ Ballio, A.; Chain, E. B.; De Leo, P.; Erlanger, B. F.; Mauri, M.; Tonolo, A. (1964). "Fusicoccin: a new wilting toxin produced by Fusicoccum amygdali". Nature. 203 (4942): 297. doi:10.1038/203297a0.
- ↑ de Boer AH, de Vries-van Leeuwen IJ (2012). "Fusicoccanes: diterpenes with surprising biological functions". Trends in Plant Science. 17 (6): 360–368. doi:10.1016/j.tplants.2012.02.007. PMID 22465041.
- ↑ Toyomasu T, Tsukahara M, Kaneko A, Niida R, Mitsuhashi W, Dairi T, Kato N, Sassa T (2007). "Fusicoccins are biosynthesized by an unusual chimera diterpene synthase in fungi". PNAS. 104 (9): 3084–3088. doi:10.1073/pnas.0608426104. PMID 17360612.
- ↑ Ono Y, Minami A, Noike M, Higuchi Y, Toyoasu T, Sassa T, Kato N, Dairi T (2011). "Dioxygenases, keyenzymes to determine the aglycon structures of fusicoccin and brassicicene, diterpene compounds produced by fungi". J. Am. Chem. Soc. 133 (8): 2548–2555. doi:10.1021/ja107785u. PMID 21299202.
- ↑ Noike M, Liu C, Ono Y, Hamano Y, Toyomasu T, Sassa T, Kato N, Dairi T (2012). "An enzyme catalyzing o-prenylation of the glucose moiety of fusicoccin A, a diterpene glucoside produced by the fungus phopopsis amygdali". ChemBioChem. 13 (4): 566–573. doi:10.1002/cbic.201100725. PMID 22287087.