Gutmann–Beckett method
The Gutmann–Beckett method is an experimental procedure used by chemists to assess the Lewis acidity of molecular species. Triethylphosphine oxide (Et3PO, TEPO) is used as a probe molecule and systems are evaluated by 31P NMR spectroscopy.
Gutmann (1975) used 31P NMR spectroscopy to parameterize Lewis acidity of solvents by Acceptor Numbers.[1] Beckett (1996) recognised its more generally utility and adapted the procedure so that it could be easily applied to molecular species dissolved in weakly Lewis acidic solvents.[2] The term Gutmann–Beckett method was first used in chemical literature in 2007.[3]
Prof. Dr Viktor Gutmann (1921–98) was an eminent Austrian chemist (see de:Viktor Gutmann) renowned for his work on non-aqueous solvents. Prof. Michael A. Beckett is Head of the School of Chemistry at Bangor University, UK.
Application to boranes
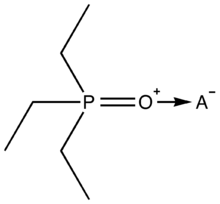
The 31P chemical shift (δ) of Et3PO is sensitive to chemical environment but can usually be found between +40 and +100 ppm. The O atom in Et3PO is a Lewis base, and its interaction with Lewis acid sites causes deshielding of the adjacent P atom. Gutmann described an Acceptor Number (AN) scale for solvent Lewis acidity [4] with two reference points relating to the 31P NMR chemical shift of Et3PO in the weakly Lewis acidic solvent hexane (δ = 41.0 ppm, AN 0) and in the strongly Lewis acidic solvent SbCl5 (δ = 86.1 ppm, AN 100). Acceptor numbers can be calculated from AN = 2.21 x (δsample – 41.0) and higher AN values indicate greater Lewis acidity. Boron trihalides are archetypal Lewis acids and have the following AN values: BF3 (89) < BCl3 (106) < BBr3 (109) < BI3 (115).[2] The Lewis acidity of other molecules can be obtained in weakly Lewis acidic solvents by 31P NMR measurements of their Et3PO adducts.[5] The Gutmann–Beckett method has been applied to Lewis acids derived fluoroarylboranes [5][6] such as B(C6F5)3 (AN 82), and borenium cations, and its application to boron compounds in general has been reviewed.[7]
Application to other compounds
The Gutmann–Beckett method has been successfully applied to main group compounds [5][8][9][10][11] (e.g. AlCl3, AN 87; silylium cations; [E(bipy)2]3+ (E = P, As, Sb, Bi) cations; cationic 4 coordinate Pv and Sbv derivatives) and transition-metal compounds [5][12] (e.g. TiCl4, AN 70) which display Lewis acidic properties.
References
- ↑ U. Mayer, V. Gutmann, and W. Gerger, "The acceptor number – a quantitative empirical parameter for the electrophilic properties of solvents", Monatshefte fur Chemie, 1975, 106, 1235–1257. doi: 10.1007/BF00913599
- 1 2 M.A. Beckett, G.C. Strickland, J.R. Holland, and K.S. Varma, "A convenient NMR method for the measurement of Lewis acidity at boron centres: correlation of reaction rates of Lewis acid initiated epoxide polymerizations with Lewis acidity", Polymer, 1996, 37, 4629–4631. doi: 10.1016/0032-3861(96)00323-0
- ↑ G.C. Welch, L.Cabrera, P.A. Chase, E. Hollink, J.M. Masuda, P. Wei, and D.W. Stephan,"Tuning Lewis acidity using the reactivity of "frustrated Lewis pairs": facile formation of phosphine-boranes and cationic phosphonium-boranes", Dalton Trans., 2007, 3407–3414. doi: 10.1039/b704417h
- ↑ V. Gutmann, "Solvent effects on reactivities of organometallic compounds", Coord. Chem. Rev., 1976, 18, 225–255. doi: 10.1016/S0010-8545(00)82045-7
- 1 2 3 4 M.A. Beckett, D.S. Brassington, S.J. Coles, and M.B. Hursthouse, "Lewis acidity of tris(pentafluorophenyl)borane: crystal and molecular structure of B(C6F5)3.OPEt3", Inorg. Chem. Commun., 2000, 3, 530–533. doi: 10.1016/S1387-7003(00)00129-5
- ↑ S.C. Binding, H. Zaher, F.M. Chadwick, and D. O'Hare, "Heterolytic activation of hydrogen using frustrated Lewis pairs containing tris(2,2',2'-perfluorobiphenyl)borane", Dalton Trans., 2012, 41, 9061–9066. doi: 10.1039/c2dt30334e
- ↑ B. Sivaev, V.L. Bregadze, “Lewis acidity of boron compounds”, Coord. Chem. Rev., 2014, 270/271, 75-88. doi: 10.1016/j.ccr2013.10.017
- ↑ H. Grossekappenberg, M. Reissmann, M. Schmidtmann, and T. Mueller, “Quantitative assessment of the Lewis acidity of silylium ions”, Organometallics, 2015, 34, 4952-4958. doi: 10.1021/acs.organomet.5b00556
- ↑ S.S. Chitnis, A.P.M. Robertson, N. Burford, B.O. Patrick, R. McDonald, and M.J. Ferguson, “Bipyridine complexes of E3+ (E = P, As, Sb, Bi): strong Lewis acids, sources of E(OTf)3 and synthons for EI and Ev cations”, Chemical Sciences, 2015, 6, 6545-6555. doi: 10.1039/C5SC02423D
- ↑ J.M. Bayne and D.W. Stephan, “Phosphorus Lewis acids: emerging reactivity and applications in catalysis”, Chem. Soc. Rev., 2015, 45, 765-774. doi:10.1039/c5cs00516g
- ↑ B. Pan and F. Gabbai, “[Sb(C6H5)4][B(C6F5)4]: an air stable Lewis acidic stibonium salt that activates strong element-fluorine bonds”, J. Am. Chem. Soc., 2014, 136, 9564-9567. doi: 10.1021/ja505214m
- ↑ C.-Y. Wu, T. Horibe, C.B. Jacobsen, and D. Toste, “Stable gold(III) catalysts by oxidative addition of a carbon-carbon bond”, Nature, 2015, 517, 449-454. doi: 10.1038/nature14104