Halomon
 | |
 | |
| Names | |
|---|---|
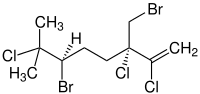
| IUPAC names
(3S,6R)-6-Bromo-3-(bromomethyl)- 2,3,7-trichloro-7-methyl-1-octene | |
| Other names
(–)-Halomon | |
| Identifiers | |
| 142439-86-9 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL136685 |
| ChemSpider | 116941 |
| PubChem | 132430 |
| |
| |
| Properties | |
| C10H15Br2Cl3 | |
| Molar mass | 401.3931 g/mol |
| Density | 1.824 g/cm3 |
| Melting point | 56 to 57 °C (133 to 135 °F; 329 to 330 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Halomon is a polyhalogenated monoterpene first isolated from the marine red algae Portieria hornemannii.[1][2] Halomon has attracted research interest because of its promising profile of selective cytotoxicity that suggests its potential use as an antitumor agent.[3]
Halomon is in a class of chemical compounds known as halocarbons, which are often potent alkylating agents which may be toxic to individual cells or to living organisms. The red algae that naturally produce halomon and other related compounds probably do so as a poisonous defense against fish or other marine life that may see it as a potential source of food. Halomon, however, is a selective toxin; studies at the National Cancer Institute have indicated that it is more toxic to certain types of tumor cells than to other cells.[1]
The algae that produces halomon is difficult to locate, identify, and collect and the concentration of halomon in the organism is extremely low. Therefore, obtaining a sufficient amount of halomon to conduct preclinical research has been difficult. Consequently, there has been active interest in developing synthetic methods in the laboratory for the preparation of halomon and related compounds.[4][5][6]
Recent research has shown that halomon and a related halogenated monoterpene may act as demethylating agents, suggesting a possible mechanism of action for the pharmacological effects of halomon.[7]
References
- 1 2 Fuller, Richard W.; Cardellina, John H., II; Kato, Yoko; Brinen, Linda S.; Clardy, Jon; Snader, Kenneth M.; Boyd, Michael R. (1992). "A pentahalogenated monoterpene from the red alga Portieria hornemannii produces a novel cytotoxicity profile against a diverse panel of human tumor cell lines". Journal of Medicinal Chemistry. 35 (16): 3007–3011. doi:10.1021/jm00094a012. PMID 1501227.
- ↑ Fuller, Richard W.; Cardellina, John H., II; Jurek, Jaroslaw; Scheuer, Paul J.; Alvarado-Lindner, Belinda; McGuire, Mary; Gray, Glenn N.; Steiner, Jorge Rios; Clardy, Jon; et al. (1994). "Isolation and Structure/Activity Features of Halomon-Related Antitumor Monoterpenes from the Red Alga Portieria hornemannii". Journal of Medicinal Chemistry. 37 (25): 4407–4411. doi:10.1021/jm00051a019. PMID 7996553.
- ↑ Egorin, Merrill J.; Sentz, Dorothy L.; Rosen, D. Marc; Ballesteros, Michael F.; Kearns, Christine M.; Callery, Patrick S.; Eiseman, Julie L. (1996). "Plasma pharmacokinetics, bioavailability, and tissue distribution in CD2F1 mice of halomon, an antitumor halogenated monoterpene isolated from the red algae Portieria hornemannii". Cancer Chemotherapy and Pharmacology. 39 (1/2): 51–60. doi:10.1007/s002800050537.
- ↑ Schlama, Thierry; Baati, Rachhid; Gouveneur, Veronique; Valleix, Alain; Flack, John R.; Mioskowski, Charles (1998). "Total synthesis of (± )-halomon by a Johnson-Claisen rearrangement". Angewandte Chemie International Edition. 37 (15): 2085–2087. doi:10.1002/(SICI)1521-3773(19980817)37:15<2085::AID-ANIE2085>3.0.CO;2-J.
- ↑ Michael E. Jung, Michael H. Parker (1997). "Synthesis of Several Naturally Occurring Polyhalogenated Monoterpenes of the Halomon Class". Journal of Organic Chemistry. 62 (21): 7094–7095. doi:10.1021/jo971371. PMID 11671809.
- ↑ Sotokawa, Takayuki; Noda, Takeshi; Pi, Sun; Hirama, Masahiro (2000). "A three-step synthesis of halomon". Angewandte Chemie. 39 (19): 3430–3432. doi:10.1002/1521-3773(20001002)39:19<3430::AID-ANIE3430>3.0.CO;2-3. PMID 11091378.
- ↑ Andrianasolo, Eric H.; France, Dennis; Cornell-Kennon, Susan; Gerwick, William H. (2006). "DNA Methyl Transferase Inhibiting Halogenated Monoterpenes from the Madagascar Red Marine Alga Portieria hornemannii". Journal of Natural Products. 69 (4): 576–579. doi:10.1021/np0503956. PMID 16643029.