Hashimoto's encephalopathy
| Hashimoto's encephalopathy | |
|---|---|
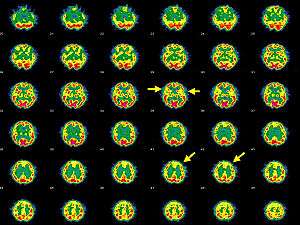 | |
| Brain SPECT transaxial images of a patient afflicted with Hashimoto's encephalopathy. | |
| Classification and external resources | |
| ICD-10 | Xxx.x |
| ICD-9-CM | xxx |
Hashimoto's encephalopathy, also known as steroid responsive encephalopathy associated with autoimmune thyroiditis (SREAT), is a neourological condition characterized by encephalopathy, thyroid autoimmunity, and good clinical response to steroids. It is associated with Hashimoto's thyroiditis. It was first described in 1966. It is sometimes referred to as a neuroendocrine disorder, although the condition's relationship to the endocrine system is widely disputed. It is recognized as a rare disease by the NIH Genetic and Rare Diseases Information Center.[1]
Up to 2005 there were almost 200 published case reports of this disease. Between 1990 and 2000 43 cases were published. Since that time, research has expanded and numerous cases are being reported by scientists around the world, suggesting that this rare condition is likely to have been significantly undiagnosed in the past. Over 100 scientific articles on Hashimoto's encephalopathy were published between 2000 and 2013.[2]
Definition
A relapsing encephalopathy occurring in association with Hashimoto's thyroiditis, with high titers of anti-thyroid antibodies. Clinically, the condition may present one or more symptoms. Onset is often gradual and may go unnoticed by the patient and close associates to the patients. Symptoms sometimes resolve themselves within days to weeks, leaving a patient undiagnosed. For many other patients, the condition may result in ongoing problems with a variety of manifestations, often confusing clinicians due to the diffuse nature of symptoms.
Signs and symptoms
The onset of symptoms tends to be fairly gradual and to occur over 1–7 days.
Symptoms of Hashimoto's encephalopathy may include:
- personality changes
- aggression
- delusional behavior
- concentration and memory problems
- coma
- disorientation
- headaches
- jerks in the muscles (myoclonus – 65% cases)
- lack of coordination (ataxia – 65% cases)
- partial paralysis on the right side
- psychosis
- seizures (60% cases)
- sleep abnormalities (55% cases)
- speech problems (transient aphasia – 80% cases)
- status epilepticus (20% cases)
- tremors (80% cases)
Pathogenesis
The mechanism of pathogenesis is not known but it has been hypothesized to be an autoimmune disorder, similar to Hashimoto's thyroiditis as its name suggests.
Consistent with this hypothesis, autoantibodies to alpha-enolase have been found to be associated with Hashimoto's encephalopathy.[3] Since enolase is the penultimate step in glycolysis, if it were inhibited (for example by being bound by autoantibodies) one would expect decreased energy production by each cell, leading to resulting atrophy of the affected organ.
This would occur most likely through each cell shrinking in size in response to the energy deficit (and/or in extreme situations from some cells dying via either apoptosis or necrosis, depending on location).[4] This may occur as a result of there not being enough ATP to maintain cellular functions: notably failure of the Na/K ATPase, resulting in a loss of the gradient to drive the Na/Ca antiporter which normally keeps Ca+
2 out of cells so that it does not build to toxic levels that will rupture cell lysosomes leading to apoptosis. An additional feature of a low energy state is failure to maintain axonal transport via Dynein/Kinesin ATPases, which in many diseases results in neuronal injury to both the brain and/or periphery.[5]
Pathology
Very little is known about the pathology of Hashimoto's encephalopathy. Post-mortem studies of some individuals have shown lymphocytic vasculitis of venules and veins in the brain-stem and a diffuse gliosis involving gray matter more than white matter.
As mentioned above, autoantibodies to alpha-enolase associated with Hashimoto's encephalopathy have thus far been the most hypothesized mechanism of injury.[3]
Diagnosis
Laboratory and radiological findings
- Increased liver enzyme levels (55% cases)
- Increased thyroid-stimulating hormone (55% cases)
- Increased erythrocyte sedimentation rate (25% cases)
Cerebrospinal fluid findings:
- Raised protein (25% cases)
- Negative for 14–3–3 protein
- May contain antithyroid antibodies
- Magnetic resonance imaging abnormalities consistent with encephalopathy (26% cases)
- Single photon emission computed tomography shows focal and global hypoperfusion (75% cases)
- Cerebral angiography is normal
Thyroid hormone abnormalities are common (>80% cases):
- subclinical hypothyroidism (35% cases)
- overt hypothyroidism (20% cases)
- hyperthyroidism (5% cases)
- euthyroid on levothyroxine (10% cases)
- euthyroid not on levothyroxine (20% cases)
Thyroid antibodies – both anti-thyroid peroxidase antibodies (anti-TPO, anti-thyroid microsomal antibodies, anti-M) and antithyroglobulin antibodies (anti-Tg) – in the disease are elevated but their levels do not correlate with the severity.
Electroencephalogram studies, while almost always abnormal (98% cases), are usually nondiagnostic. The most common findings are diffuse or generalized slowing or frontal intermittent rhythmic delta activity. Prominent triphasic waves, focal slowing, epileptiform abnormalities, photoparoxysmal and photomyogenic responses may be seen.
Differential diagnosis
- Alzheimer's disease
- Cerebrovascular accidents (stroke)
- Creutzfeldt–Jakob disease (CJD)
- Epilepsy
- Migraine (including basilar, hemiplegic, and retinal types)
- Other forms of autoimmune encephalitis, including forms of limbic encephalitis such as anti-NMDA receptor encephalitis
- Schizophrenia
- Spontaneous cerebrospinal fluid leak
- Viral encephalitis
Treatment
Because most patients respond to steroids or immunosuppressant treatment, this condition is now also referred to as steroid-responsive encephalopathy.
Initial treatment is usually with oral prednisone (50–150 mg/day) or high-dose IV methylprednisolone (1 g/day) for 3–7 days. Thyroid hormone treatment is also included if required.
Failure of some patients to respond to this first line treatment has produced a variety of alternative treatments including azathioprine, cyclophosphamide, chloroquine, methotrexate, periodic intravenous immunoglobulin and plasma exchange. There have been no controlled trials so the optimal treatment is not known.
Seizures, if present, are controlled with typical antiepileptic agents.
Prognosis
Duration of treatment is usually between 2 and 25 years. Earlier reports suggested that 90% of cases stay in remission after discontinuation of treatment; however, this is at odds with more recent studies which suggest that relapse commonly occurs after initial high-dose steroid treatment.[6][7] Left untreated, this condition can result in coma and death.
Epidemiology
The prevalence has been estimated to be 2.1/100,000[8] with a male to female ratio of 1:4. The mean age of onset is 44 with 20% of cases presenting before the age of 18 years. Most reported cases occur during the patient's fifth decade of life.
History
The first case of HE was described by Brain et al. in 1966.[9] The patient was a 48-year-old man with hypothyroidism, multiple episodes of encephalopathy, stroke-like symptoms and Hashimoto's thyroiditis confirmed by elevated anti-thyroid antibodies.
Organizations
- Autoimmune Encephalitis Alliance, a 501(c)(3) non-profit organization created in 2012, based in Durham, North Carolina
- Hashimoto's Encephalopathy SREAT Alliance, a 501(c)(3) nonprofit organization created in 2012
Alternative names
- Steroid-responsive encephalopathy associated with autoimmune thyroiditis, SREAT
- Nonvasculitic autoimmune meningoencephalitis, NAIM
- Encephalopathy associated with autoimmune thyroid disease, EAATD
References
- ↑ "Hashimoto's encephalitis - Disease - Overview". Genetic and Rare Diseases Information Center (GARD).
- ↑ "Scientific Research/Articles – Articles Published in 2014". hesaonline.org.
- 1 2 Fujii A, Yoneda M, Ito T, Yamamura O, Satomi S, Higa H, Kimura A, Suzuki M, Yamashita M, Yuasa T, Suzuki H, Kuriyama M (May 2005). "Autoantibodies against the amino terminal of alpha-enolase are a useful diagnostic marker of Hashimoto's encephalopathy". J. Neuroimmunol. 162 (1–2): 130–6. doi:10.1016/j.jneuroim.2005.02.004. PMID 15833368.
- ↑ Shigeomi Shimizu2, Yutaka Eguchi, Wataru Kamiike, Yuko Itoh, Jun-ichi Hasegawa, Kazuo Yamabe, Yoshinori Otsuki, Hikaru Matsuda, and Yoshihide Tsujimoto The First Department of Surgery. Department of Medical Genetics. BiomedicalResearch Center. Osaka University Medical School, 2-2 Yatnadfioka, Sunti 56.5. Japan, and Depannient of Anatomy and Biology. Osaka Medical College. Japan.| url=http://cancerres.aacrjournals.org/content/56/9/2161.full.pdf
- ↑ Ihejirika DF. PASS Program Course Notes: USMLE Preparation. Lulu.com; 2014.
- ↑ Castillo P, Woodruff B, Caselli R, et al. (February 2006). "Steroid-responsive encephalopathy associated with autoimmune thyroiditis". Archives of Neurology. 63 (2): 197–202. doi:10.1001/archneur.63.2.197. PMID 16476807.
- ↑ Flanagan EP, McKeon A, Lennon VA, et al. (October 2010). "Autoimmune dementia: clinical course and predictors of immunotherapy response". Mayo Clinic Proceedings. 85 (10): 881–97. doi:10.4065/mcp.2010.0326. PMC 2947960
 . PMID 20884824.
. PMID 20884824. - ↑ Ferracci F, Bertiato G, Moretto G (February 2004). "Hashimoto's encephalopathy: epidemiologic data and pathogenetic considerations". Journal of the Neurological Sciences. 217 (2): 165–8. doi:10.1016/j.jns.2003.09.007. PMID 14706219.
- ↑ Brain L, Jellinek EH, Ball K (September 1966). "Hashimoto's disease and encephalopathy". Lancet. 2 (7462): 512–4. doi:10.1016/S0140-6736(66)92876-5. PMID 4161638.
External links
- Schiess N, Pardo CA (October 2008). "Hashimoto's encephalopathy". Annals of the New York Academy of Sciences. 1142: 254–65. doi:10.1196/annals.1444.018. PMID 18990131.
- Taylor SE, Garalda ME, Tudor-Williams G, Martinez-Alier N (February 2003). "An organic cause of neuropsychiatric illness in adolescence". Lancet. 361 (9357): 572. doi:10.1016/S0140-6736(03)12517-2. PMID 12598143.