Hemithioacetal
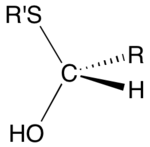
Hemithioacetal is an organic functional group with the general formula RCH(OH)SR. They are also called thiohemiacetal. With four substituents on carbon, hemithioacetals are chiral. A related functional group are dithiohemiacetal, with the formula RCH(SH)SR.[1] Although they can be important intermediates, hemithioacetals are usually not isolated since they exist in equilibrium with the thiol and aldehyde:
Formation and structure
Hemithioacetal form by the reaction of a thiol and an aldehyde:
- RCHO + R’SH ⇌ RCH(OH)(SR’)
Hemithioacetals usually arise via acid catalysis. They typically are intermediates in the formation of dithioacetals:
- RCH(OH)(SR’) + R’SH ⇌ RCH(SR’)2 + H2O
Isolable hemithioacetal
S.png)
Hemithioacetals ordinarily readily dissociate into thiol and aldehyde, however some have been isolated. In general these isolable hemithioacetals are cyclic, which disfavors dissociation, and can often be further stabilized by the presence of acid.[2] An important class are S-glycosides, such as octylthioglucoside, which are formed by a reaction between thiols and sugars. Other examples include 2-hydroxytetrahydrothiophene[3] and the anti-HIV drug Lamivudine.[4] Another class of isolable hemithioacetals are derived from carbonyl groups that form stable hydrates. For example, thiols react with hexafluoroacetone trihydrate to give hemithioacetals, which can be isolated.[5]
Hemithioacetals in nature
Glyoxalase I, which is part of the glyoxalase system present in the cytosol, catalyzes the conversion of α-oxoaldehyde (RC(O)CHO) and the thiol glutathione (abbreviated GSH) to S-2-hydroxyacylglutathione derivatives [RCH(OH)CO-SG]. The catalytic mechanism involves an intermediate hemithioacetal adduct [RCOCH(OH)-SG]. The spontaneous reaction forms methylglyoxal-glutathione hemithioacetal and human glyoxalse I.[6]
A hemithioacetal is also invoked in the mechanism of prenylcysteine lyase. In catalytic mechanism, S-farnesylcysteine is oxidized by a flavin to a thiocarbenium ion. The thiocarbenium ion hydrolyzes to form the hemithioacetal:
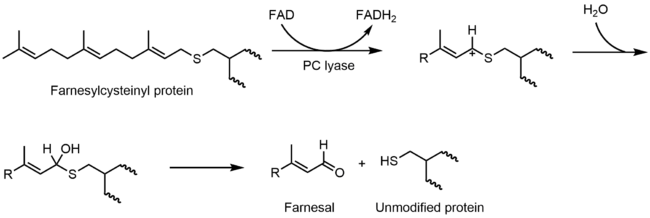
After formation, the hemithioacetal breaks into hydrogen peroxide, farnesal, and cysteine.[7]
References
- ↑ http://goldbook.iupac.org/T06355.html
- ↑ Barnett, Ronald E.; Jencks, William P. (November 1969). "Diffusion-controlled and concerted base catalysis in the decomposition of hemithioacetals". Journal of the American Chemical Society. 91 (24): 6758–6765. doi:10.1021/ja01052a038.
- ↑ Cox, J. M.; Owen, L. N. (1967). "Cyclic hemithioacetals: analogues of thiosugars with sulphur in the ring". Journal of the Chemical Society C: Organic: 1130. doi:10.1039/J39670001130.
- ↑ Milton, John; Brand, Stephen; Jones, Martin F.; Rayner, Christopher M. (September 1995). "Enantioselective enzymatic synthesis of the anti-viral agent lamivudine (3TC™)". Tetrahedron Letters. 36 (38): 6961–6964. doi:10.1016/0040-4039(95)01380-Z.
- ↑ Field, Lamar; Sweetman, B. J.; Bellas, Michael (July 1969). "Biologically oriented organic sulfur chemistry. II. Formation of hemimercaptals or hemimercaptoles (.alpha.-hydroxy sulfides) as a means of latentiating thiols". Journal of Medicinal Chemistry. 12 (4): 624–628. doi:10.1021/jm00304a014.
- ↑ Thornalley, PJ (December 2003). "Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation.". Biochemical Society Transactions. 31 (Pt 6): 1343–8. doi:10.1042/bst0311343. PMID 14641060.
- ↑ Digits, J. A.; Pyun, H.-J.; Coates, R. M.; Casey, P. J. (16 August 2002). "Stereospecificity and Kinetic Mechanism of Human Prenylcysteine Lyase, an Unusual Thioether Oxidase". Journal of Biological Chemistry. 277 (43): 41086–41093. doi:10.1074/jbc.M208069200. PMID 12186880.