Hexadehydro Diels-Alder reaction
In organic chemistry, the hexadehydro-Diels-Alder (HDDA) reaction is an organic chemical reaction between a diyne (2 alkyne functional groups arranged in a conjugated system) and an alkyne to form a reactive benzyne species, via a [4+2] cycloaddition reaction.[1][2][3] This benzyne intermediate then reacts with a suitable trapping agent to form a substituted aromatic product. This reaction is a derivative of the established Diels-Alder reaction and proceeds via a similar [4+2] cycloaddition mechanism. The HDDA reaction is particularly effective for forming heavily functionalized aromatic systems and multiple ring systems in one synthetic step.

Reaction Mechanism
Depending on the substrate chosen, the HDDA reaction can be initiated thermally or by the addition of a suitable catalyst, often a transition metal.[1][2][4][5] The prevailing mechanism for the thermally-initiated HDDA reaction is a [4+2] cycloaddition between a conjugated diyne (1,3-dialkyne) and an alkyne (often referred to as a “diynophile” in analogy to the Diels-Alder “dienophile”) to form an ortho-benzyne species.[1][2] The metal-catalyzed HDDA is thought to proceed through a similar pathway, forming a metal-stabilized benzyne, which is then trapped.
The simplest model of an HDDA reaction is the cycloaddition of butadiyne and acetylene to form ortho-benzyne (o-benzyne, shown below).[6] This reactive intermediate (denoted by brackets) subsequently reacts with a generalized trapping reagent that consists of a nucleophilic (Nu-) and electrophilic (El-) site, giving the benzenoid product shown.

The o-benzyne intermediate can be visualized in the two resonance (chemistry) forms illustrated above. The most commonly depicted form is the alkyne (1), but the cumulene (1’) form can be helpful in visualizing ring formation by [4+2] cycloaddition.
Thermodynamics and Kinetics
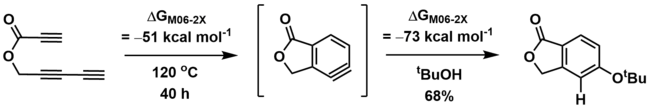
The HDDA reaction is often thermodynamically favorable (exothermic), but can have a significant kinetic barrier to reaction (high activation energy). Calculations have suggested that the formation of unsubstituted o-benzyne (from butadiyne and acetylene, above) has an activation energy of 36 kcal mol−1, but is thermodynamically favorable, estimated to be exothermic by -51 kcal mol−1.[6] As a result of higher activation energy, some HDDA reactions require heating to elevated temperatures (>100 ⁰C) in order to initiate.[1][2]
Furthermore, the benzyne trapping step is also thermodynamically downhill, calculated to be an additional -73 kcal mol−1 for trapping of an ester-substituted o-benzyne with tert-butanol.[1]
The HDDA [4+2] cycloaddition can occur via either a concerted pathway or a stepwise reaction, diradical pathway. These two pathways can differ in activation energy depending on substrate and reaction system. Computational studies have suggested that while both pathways are comparable in activation energy for unactivated (unsubstituted) diynophiles, the stepwise pathway has a lower activation energy barrier, and so is the dominant pathway, for activated diynophiles.[6][7]
Regiochemistry
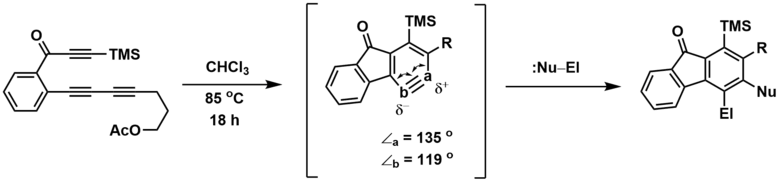
The regiochemistry of non-symmetrical HDDA-derived benzyne trapping can be explained by a combination of electronic and ring distortion effects.[1] Computationally, the more obtuse angle (a) corresponds to the more electron deficient (δ+) benzyne carbon, leading to attack of the nucleophilic component at this site. Consequently, the electrophilic component adds at the more electron rich (δ-) site (b).
Terminology
The HDDA reaction is a derivative of, and mechanistically related to the classical Diels-Alder reaction. As described by Hoye and coworkers, the HDDA reaction can be viewed conceptually as a member of a series of pericyclic reactions with increasing unsaturation (by incremental removal of hydrogen pairs).[1] The “hexadehydro” descriptor is derived from this interpretation, as the simplest HDDA reaction product (o-benzyne, 4 hydrogens) has 6 fewer hydrogen atoms than the simplest Diels-Alder reaction product (cyclohexene, 10 hydrogens).

Formally, the hexadehydro Diels-Alder reaction describes only the formation of the benzyne, but this species is an unstable intermediate that reacts readily with a variety of trapping partners, including reaction solvents. Thus, in practice the HDDA reaction describes a two-step cascade reaction of benzyne formation and trapping to yield the final product.
Historical Development
The first examples of the HDDA reaction were reported independently in 1997 by the groups of Ueda and Johnson.[2][8][9][10] Johnson and co-workers observed the cyclization of 1,3,8-nonatriyne under flash vacuum thermolysis (600 °C, 10−2 torr) to form two products, indane and the dehydrogenation product indene, in 95% combined yield. Deuterium labeling studies suggested that the product was formed by a [4+2] cycloaddition to a benzyne intermediate, followed by in-situ reduction to form the observed products.[8] Ueda and co-workers observed that acyclic tetraynes cyclized at room temperature to form 5H-fluorenol derivatives. The formation of a benzyne intermediate was determined by trapping studies, using benzene or anthracene to trap the benzyne as a Diels-Alder adduct.[10] Ueda and co-workers further elaborated on this method in subsequent reports, trapping the benzyne using a variety of nucleophiles (oxygen, nitrogen, and sulfur-based), as well as synthesizing larger, fused-ring aromatic systems.[11][12][13][14][15]
While known for over a decade, the HDDA reaction did not come into wider synthetic use until 2012, when Hoye and co-workers conducted a thorough investigation into the scope and utility of this cycloaddition.[1] That paper referred to this diyne-diynophile reaction as the “hexadehydro Diels-Alder (HDDA)” reaction, and this terminology has since come into more widespread use. Since 2012, the HDDA reaction has been an area of renewed interest and has attracted further study by a number of research groups.[4][5][7][16]
Reaction Scope
One of the main advantages of the HDDA reaction over other methods of accessing benzynes is the simplicity of the reaction system. HDDA reaction of triynes or tetraynes forms benzynes without the direct formation of by-products. In comparison, the formation of benzyne through removal of ortho-substituents on arenes results in stoichiometric amounts of byproducts from those substituents. For example, formation of benzyne from 1 mole of 2-trimethylsilylphenyl trifluoromethanesulfonate (triflate) produces 1 mole of trimethylsilyl fluoride and 1 mole of triflate ion. Byproducts can compete with other reagents for benzyne trapping, cause side-reactions, and may require additional purification.
Additionally, the HDDA reaction can be useful for substrates with sensitive functionality that might not be tolerated by other benzyne formation conditions (e.g. strong base). The thermally-initiated HDDA reaction has been shown to tolerate esters, ketones, protected amides, ethers, protected amines, aryl halides, alkyl halides, alkenes, and cyclopropanes.[1][4][17]
Green Chemistry
The HDDA reaction can fulfill several principles of green chemistry.
- Atom Economy – All of the atoms in the HDDA substrate remain in the product after the reaction and atoms of the trapping reagent are incorporated into the product.
- Reduced Waste – Formation of the benzyne species produces no stoichiometric byproducts. Products are often formed in high yield with few side-products.
- Catalysis – HDDA reaction occurs thermally or with a sub-stoichiometric amount of catalyst.
Synthetic Applications
Intramolecular Trapping
The HDDA reaction can be used to synthesize multi-cyclic ring systems from linear precursors containing the diyne, diynophile, and the trapping group. For example, Hoye and co-workers were able to synthesize fused, tricyclic ring systems from linear triyne precursors in one step and high yields via a thermally-initiated, intramolecular HDDA reaction.[1] Furthermore, both nitrogen- and oxygen-containing heterocycles could be incorporated by use of an appropriate precursor. In this case, the pendant silyl ether provided the trapping group, through a retro-Brook rearrangement.

Intermolecular Trapping
HDDA-generated benzynes can also be trapped intermolecularly by a variety of trapping reagents. Careful choice of trapping reagent can add further functionality, including aryl halides, aryl heteroatoms (phenols and aniline derivatives), and multiple ring systems.[1][18]

Ene Reactions
The HDDA reaction can be used in a cascade reaction sequence with ene reactions, such as the Alder ene reaction and the aromatic ene reaction.[16][19] The HDDA-generated benzyne can be trapped with a suitable ene donor that is covalently tethered to the benzyne. The benzyne serves as the enophile, while the ene can be an alkene (Alder ene) or an aromatic ring (aromatic ene). Lee and co-workers have shown an HDDA-Alder ene cascade reaction that can produce a variety of products, including medium-sized fused rings, spirocycles, and allenes.[16]
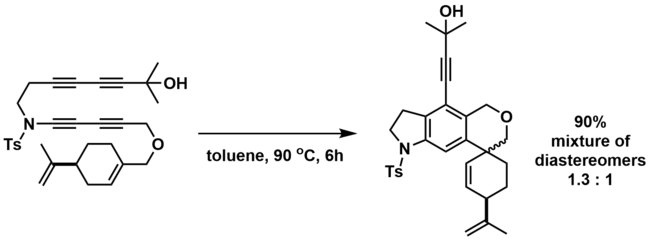
Hoye and co-workers demonstrated a thermally-initiated triple HDDA-aromatic ene-Alder ene cascade that leads to heavily-functionalized products in one-step with no additional reagents or by-products.[19]

Dehydrogenation
HDDA-derived benzynes have also been shown to dehydrogenate saturated alkanes to form alkenes.[20] In the absence of external trapping reagents, the benzyne intermediate can abstract vicinal (chemistry) hydrogen atoms from a suitable donor, often the reaction solvent (such as tetrahydrofuran or cyclooctane). This desaturates the donor alkane, forming an alkene, and traps the benzyne to a dihydrobenzenoid product. Isotopic labelling and computational studies suggest that the double hydrogen transfer mechanism occurs by a concerted pathway and that the rate of reaction is highly dependent on the conformation of the alkane donor.[20] This reaction can be used to access 1,2,3,4-tetrasubstituted aromatic rings, a substitution pattern that can be difficult to access through other synthetic methodology.

C-H Activation
The HDDA reaction can also be used as a method of C-H activation, where a pendant alkane C-H bond traps a metal-complexed aryne intermediate. Lee and co-workers observed that transition metal catalysts induced an HDDA reaction of tetraynes that was intramolecularly trapped by a pendant, sp3 C-H bond.[4] Primary, secondary, and tertiary C-H bonds were all reactive trapping partners, with silver salts being the most effective catalysts. Deuterium labelling experiments suggest that the (sp3) C-H bond breaking and (sp2) C-H bond forming reactions occur in a concerted fashion.
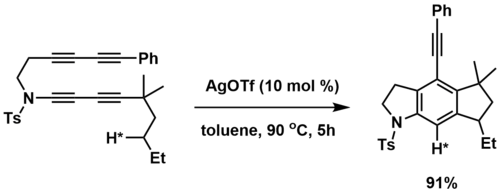
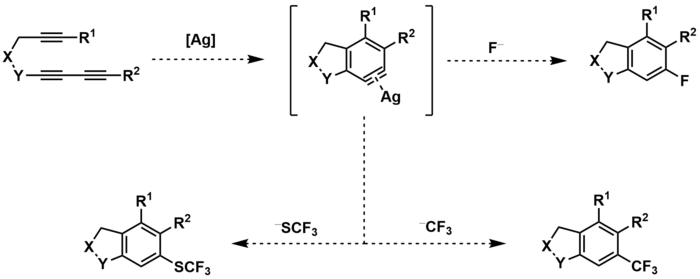
Fluorination
The silver-catalyzed HDDA reaction has also been used to synthesize organofluorine compounds by use of a fluorine-containing counterion.[17] The metal-complexed aryne intermediate can be trapped by the counterion to produce aryl rings with fluoro, trifluoromethyl, or trifluoromethylthiol substituents. Unstable counterions, such as CF3−, can be produced in-situ.
References
- 1 2 3 4 5 6 7 8 9 10 11 Hoye, T. R.; Baire, B.; Niu, D.; Willoughby, P. H.; Woods, B. P. Nature, 2012, 490, 208
- 1 2 3 4 5 Holden, C.; Greaney, M. F. Angew. Chem. Int. Ed. Engl., 2014, 53, 5746
- ↑ Yeoman, J. T. S.; Reisman, S. E. Nature, 2012, 490, 179
- 1 2 3 4 Yun, S. Y.; Wang, K.-P.; Lee, N.-K.; Mamidipalli, P.; Lee, D. J. Am. Chem. Soc., 2013, 135, 4668
- 1 2 Vandavasi, J. K.; Hu, W.-P.; Hsiao, C.-T.; Senadi, G. C.; Wang, J.-J. RSC Adv., 2014, 4, 57547
- 1 2 3 Ajaz, A.; Bradley, A. Z.; Burrell, R. C.; Li, W. H. H.; Daoust, K. J.; Bovee, L. B.; DiRico, K. J.; Johnson, R. P. J. Org. Chem., 2011, 76, 9320
- 1 2 Liang, Y.; Hong, X.; Yu, P.; Houk, K. N. Org. Lett., 2014, 16, 5702
- 1 2 Bradley, A. Z.; Johnson, R. P. J. Am. Chem. Soc., 1997, 119, 9917
- ↑ Cahill, K. J.; Ajaz, A.; Johnson, R. P. Aust. J. Chem., 2010, 63, 1007
- 1 2 Miyawaki, K.; Suzuki, R.; Kawano, T.; Ueda, I. Tetrahedron Lett., 1997, 38, 3943
- ↑ Ueda, I.; Sakurai, Y.; Kawano, T.; Wada, Y.; Futai, M. Tetrahedron Lett., 1999, 40, 319
- ↑ Miyawaki, K.; Kawano, T.; Ueda, I. Tetrahedron Lett., 2000, 41, 1447
- ↑ K. Miyawaki, F. Ueno, I. Ueda, Heterocycles, 2001, 54, 887
- ↑ Torikai, K.; Otsuka, Y.; Nishimura, M.; Sumida, M.; Kawai, T.; Sekiguchi, K.; Ueda, I. Bioorg. Med. Chem., 2008, 16, 5441
- ↑ Kimura, H.; Torikai, K.; Miyawaki, K.; Ueda, I. Chem. Lett., 2008, 37, 662
- 1 2 3 Karmakar, R.; Mamidipalli, P.; Yun, S. Y.; Lee, D. Org. Lett., 2013, 15, 1938
- 1 2 Wang, K.-P.; Yun, S. Y.; Mamidipalli, P.; Lee, D. Chem. Sci., 2013, 4, 3205
- ↑ Niu, D.; Wang, T.; Woods, B. P.; Hoye, T. R. Org. Lett., 2014, 16, 254
- 1 2 Niu, D.; Hoye, T. R. Nat. Chem., 2014, 6, 34
- 1 2 Niu, D.; Willoughby, P. H.; Woods, B. P.; Baire, B.; Hoye, T. R. Nature, 2013, 501, 531