Hydrazine sulfate
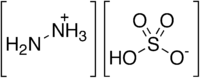 | |
| Names | |
|---|---|
| IUPAC name
Hydrazinium hydrogen sulfate, hydrazinium sulfate | |
| Identifiers | |
| 10034-93-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 23225 |
| ECHA InfoCard | 100.030.088 |
| EC Number | 233-110-4 |
| PubChem | 24842 |
| UNII | 1N369SAT01 |
| |
| |
| Properties | |
| H6N2O4S | |
| Molar mass | 130.12 g·mol−1 |
| 30 g/L (20°C) | |
| Hazards | |
| Safety data sheet | External MSDS |
| EU classification (DSD) |
|
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Hydrazine sulfate is the salt of hydrazine and sulfuric acid. It is a white, water-soluble salt of the cation hydrazinium and the anion bisulfate, giving the formula (N2H5)HSO4. It is prepared by treating an aqueous solution of hydrazine with sulfuric acid. It has a number of laboratory uses in analytical chemistry and the synthesis of organic compounds. Hydrazine sulfate is used as a precursor to hydrazine.
For some applications, this salt is preferred over pure hydrazine because it is nonvolatile and less susceptible to atmospheric oxidation on storage. These same properties make it the preferred form of hydrazine for dietary supplements and pharmaceutical trials. It is relatively inexpensive, with 100 grams of analytical grade hydrazine sulfate costing about US$20, and 100 tablets or capsules (60 milligrams hydrazine sulfate) costing US$20 to US$60. Hydrazine sulfate also has a variety of uses in the chemical industry.[1] This salt once attracted attention for its alleged medicinal properties.
Preparation
The salt is prepared by oxidation of ammonium sulfate with bleach. Hydrazine sulfate is purified by recrystallization from water.[2]
If free base hydrazine is available, its solutions react with sulfuric acid to give the salt.
Medicinal applications
Known by the trade name Sehydrin, it is a chemical compound that has been used as an alternative medical treatment for the loss of appetite (anorexia) and weight loss (cachexia), which are often associated with cancer.[3][4][5][6] Hydrazine sulfate has never been approved in the United States as safe and effective in treating any medical condition, although it is marketed as a dietary supplement.[7] It is also sold over the Internet by websites that promote its use as a cancer therapy.[8] The active ingredient is hydrazine, and the sulfate component is present to aid in formulation.
The main proponent of hydrazine sulfate as an anti-cancer agent is a U.S. physician named Joseph Gold, who developed the treatment in the mid-1970s. The use of hydrazine sulfate as a cancer remedy was popularized by the magazine Penthouse in the mid-1990s, when Kathy Keeton, wife and business partner of the magazine's publisher Bob Guccione, used it in an attempt to treat her metastatic breast cancer.[9] Alternative medicine nutritionist Gary Null wrote three of the articles about alternative cancer treatments, including one titled "The Great Cancer Fraud."[10] Keeton (until her death in 1997) and other supporters of hydrazine sulfate treatment accused the U.S. National Cancer Institute (NCI) of deliberately hiding the beneficial effects of the compound, and threatened to launch a class action lawsuit.[11][12] The NCI denied the claims,[13] and says that there is little to no evidence that hydrazine sulfate has any beneficial effects whatsoever.[7] The position of the NCI was supported by an inquiry held by the General Accounting Office.[14]
A review of the clinical research concluded that hydrazine sulfate has never been shown to act as an anticancer agent; patients do not experience remissions or regressions of their cancer, and patients do not live longer than non-treated patients.[7][15][16] Some academic reviews of alternative cancer treatments have described the compound as a "disproved and ineffective treatment for cancer".[17][18]
Industrial uses
Hydrazine sulfate also has a variety of uses in industrial chemistry, including as a chemical intermediate, as a catalyst in making fibers out of acetate, as a fungicide, antiseptic, in the analysis and synthesis of minerals and testing for arsenic in metals.[1]
Background
Hydrazine sulfate was specifically developed as a result of a proposal by Joseph Gold for a therapy that could offset the rapid loss of weight that occurs in cancer (cancer cachexia). This hypothesis was based on the fact that cancer cells are often unusually dependent on glycolysis for energy (the Warburg effect), Gold proposed that the body might offset this increased glycolysis using gluconeogenesis, which is the pathway that is the reverse of glycolysis. Since this process would require a great deal of energy, Gold thought that inhibiting gluconeogenesis might reverse this energy requirement and be an effective treatment for cancer cachexia.[19] Hydrazine is a reactive chemical that in the test tube can inactivate one of the enzymes needed for gluconeogenesis, phosphoenolpyruvate carboxykinase (PEP-CK). It was also postulated that if tumor energy gain (glycolysis) and host-energy loss (gluconeogenesis) were functionally interrelated, inhibition of gluconeogenesis at PEP CK could result in actual tumor regression in addition to reversal or arrest of cancer cachexia.[20] In this model, hydrazine sulfate is therefore thought to act by irreversibly inhibiting the enzyme phosphoenolpyruvate carboxykinase.
Clinical trials
Joseph Gold, the developer and principal proponent of hydrazine sulfate, has published several papers arguing that the compound is an effective cancer treatment.[15][21] These data have been questioned by the American Cancer Society and other investigators have been unable to repeat or confirm these results.[15][22] Gold is reported not to trust the motives or results of other investigators, with CNN quoting him as stating that "they've been out to get hydrazine sulfate, and I don't know why".[23]
In response to these results, an uncontrolled clinical trial was carried out at the Petrov Research Institute of Oncology in St. Petersburg over a period of 17 years,[15][24] and a controlled trial was carried out at the Harbor-UCLA Medical Center in California over period of 10 years, respectively. The Russian trial reported complete tumor regression in about 1% of cases, a partial response in about 3% of cases and some subjective improvement of symptoms in about half of the patients.[24] The National Cancer Institute analysis of this trial notes that interpretation of these data is difficult, due to the absence of controls, the lack of information on prior treatment and the study's reliance on subjective assessments of symptoms (i.e. asking patients if the drug had made them feel any better).[25] Overall, the trials in California saw no statistically significant effect on survival from hydrazine sulfate treatment, but noted increased calorie intake in treated patients versus controls.[26] The authors also performed a post-hoc analysis on one or more subgroups of these patients, which they reported as suggesting a beneficial effect from treatment. The design and interpretation of this trial, and in particular the validity of this subgroup analysis, was criticized in detail in an editorial in the Journal of Clinical Oncology.[27]
Later randomized controlled trials trials failed to find any improvement in survival,[28][29] For example, in a trial of the treatment of advanced lung cancer, with either cisplatin and vinblastine or these drugs plus hydrazine sulfate, saw complete tumor regression in 4% of the hydrazine group, versus 3% in the control group, and tumor progression in 36% of the hydrazine group, versus 30% of the control group; however, none of these differences were statistically significant.[30] Some trials even found both significantly decreased survival and significantly poorer quality of life in those patients receiving hydrazine sulfate.[31] These consistently negative results have resulted in hydrazine sulfate being described as a "disproven cancer therapy" in a recent medical review.[18] Similarly, other reviews have concluded that there is "strong evidence" against the use of hydrazine sulfate to treat anorexia or weight loss in cancer patients.[32][33]
Side effects
Hydrazine sulfate is toxic and carcinogenic.[34][35] Nevertheless, the short-term side effects reported in various clinical trials are relatively mild:[8] minor nausea and vomiting, dizziness and excitement, polyneuritis (inflammation of the nerves) and difficulties in fine muscle control (such as writing). However, more serious, even fatal side effects have been reported in rare cases: one patient developed fatal liver and kidney failure,[36] and another developed serious symptoms of neurotoxicity.[37] These side effects and other reports of hydrazine toxicity[26][27] are consistent with the hypothesis that hydrazine may play a role in the toxicity of the antibiotic isoniazid, which is thought to be metabolized to hydrazine in the body.[8]
Hydrazine sulfate is also a monoamine oxidase inhibitor (MAOI),[38] and is incompatible with alcohol, tranquilizers and sleeping pills (benzodiazepines and barbiturates), and other psycho-active drugs, with pethidine (meperidine, Demerol), and with foods containing significant amounts of the amino acid breakdown product tyramine, such as aged cheeses, raisins, avocados, processed and cured fish and meats, fermented products, and others.
References
- 1 2 Milne, George W. A. (2005). Gardner's commercially important chemicals: synonyms, trade names, and properties. New York: Wiley-Interscience. pp. 325. ISBN 0-471-73518-3.
- ↑ Adams, Roger; Brown, B. K. (1922). "Hydrazine sulfate". Org. Synth. 2: 37.; Coll. Vol., 1, p. 309
- ↑ Chlebowski, R. T.; Bulcavage, L.; Grosvenor, M.; et al. (1987), "Hydrazine Sulfate in Cancer Patients With Weight Loss. A Placebo-Controlled Clinical Experience", Cancer, 59 (3): 406–10, doi:10.1002/1097-0142(19870201)59:3<406::AID-CNCR2820590309>3.0.CO;2-W, PMID 3791153.
- ↑ Brauer, M.; Inculet, R. I.; Bratnager, G.; Marsh, G. D.; Driedger, A. A.; Thompson, R. T. (1994), "Insulin Protects against Hepatic Bioenergetic Deterioration by Cancer Cachexia. An in-Vivo 31P Magnetic Resonance Study", Cancer Research, 54 (24): 6383–86, PMID 7987832.
- ↑ Chlebowski, R. T.; Bulcavage, L.; Grosvenor, M.; Oktay, E.; Block, J. B.; Chlebowski, J. S.; Ali, I.; Elashoff, R. (1990), "Hydrazine Sulfate Influence on Nutritional Status and Survival in Non-Small-Cell Lung Cancer", Journal of Clinical Oncology, 8 (8): 9–15, PMID 1688616.
- ↑ Gold, J. (1999), "Long term complete response in patient with advanced, localized NSCLC with hydrazine sulfate, radiation and Carboplatin, refractory to combination chemotherapy", Proceedings of the American Association for Cancer Research (40): 642. Abstract.
- 1 2 3 Questions and answers about hydrazine sulfate, National Cancer Institute, March 12, 2009
- 1 2 3 Black, M.; Hussain, H. (2000), "Hydrazine, Cancer, the Internet, Isoniazid, and the Liver" (PDF), Annals of Internal Medicine, 133 (11): 911–13, doi:10.7326/0003-4819-133-11-200012050-00016, PMID 11103062.
- ↑ London, William M. (July 23, 2006), Penthouse's promotion of hydrazine sulfate
- ↑ Null's articles on alternative cancer therapies in Penthouse include:
- Null, Gary; Robert Houston (1979). "The Great Cancer Fraud". Penthouse: 76–78, 82, 268, 270, 272, 274, 276–278.
- Null, Gary; A. Pitrone (1980). "Suppression of new cancer therapies: Dr. Joseph Gold and hydrazine sulfate". Penthouse: 97–98, 160, 162–163.
- Null, Gary; L. Steinman (1980). "The politics of cancer. Part five. Suppression of new cancer therapies: Dr. Lawrence Burton". Penthouse: 75–76, 188–194, 196–197.
- ↑ Goldberg, Burton (June 12, 2000), Holding the National Cancer Institute Accountable for Cancer Deaths
- ↑ Goldberg, Burton; Trivieri, Larry; Anderson, John W., eds. (2002), Alternative medicine: the definitive guide (2nd ed.), Celestial Arts, pp. 50–51, 598, ISBN 1-58761-141-4.
- ↑ Jenks, S. (1993), "Hydrazine Sulfate Ad Is "Offensive"", Journal of the National Cancer Institute, 85 (7): 528–29, doi:10.1093/jnci/85.7.528, PMID 8455198.
- ↑ Nadel, M. V. (September 1995), "Cancer Drug Research—Contrary to Allegations, Hydrazine Sulfate Studies Were Not Flawed", Report to the Chairman and Ranking Minority Member, Human Resources and Intergovernmental Relations Subcommittee, House Committee on Government Reform and Oversight (PDF), Washington, D.C.: General Accounting Office, Document No. HEHS-95-141.
- 1 2 3 4 Kaegi, Elizabeth (1998), "Unconventional therapies for cancer: 4. Hydrazine sulfate. Task Force on Alternative Therapies of the Canadian Breast Cancer Research Initiative", Canadian Medical Association Journal, 158 (10): 1327–30, PMC 1229327
 , PMID 9614826.
, PMID 9614826. - ↑ Green, Saul (1997), "Hydrazine sulfate: is it an anticancer agent?", Scientific Review of Alternative Medicine, 1: 19–21
- ↑ Hydrazine sulfate / Hydrazine sulphate from the British Columbia Cancer Agency
- 1 2 Vickers A (2004), "Alternative cancer cures: "unproven" or "disproven"?", CA Cancer J Clin, 54 (2): 110–8, doi:10.3322/canjclin.54.2.110, PMID 15061600
- ↑ Gold, J. (1968), "Proposed Treatment of Cancer by Inhibition of Gluconeogenesis", Oncology, 22 (2): 185–207, doi:10.1159/000224450, PMID 5688432.
- ↑ Gold, J. (1974), "Cancer Cachexia and Gluconeogenesis", Annals of the New York Academy of Sciences, 230 (1 Paraneoplasti): 103–10, doi:10.1111/j.1749-6632.1974.tb14440.x, PMID 4522864.
- ↑ Gold J (1987), "Hydrazine sulfate: a current perspective", Nutr Cancer, 9 (2-3): 59–66, doi:10.1080/01635588709513912, PMID 3104888
- ↑ "Editorial: Unproven methods of cancer management: hydrazine sulfate", CA Cancer J Clin, 26 (2): 108–10, 1976, doi:10.3322/canjclin.26.2.108, PMID 816429
- ↑ Elizabeth Cohen Regulators warn about online cancer 'cures' CNN December 5, 2000
- 1 2 Filov, V. A.; Gershanovich, M. L.; Danova, L. A.; Ivin, B. A. (1995), "Experience of the Treatment with Sehydrin (Hydrazine Sulfate, HS) in the Advanced Cancer Patients", Investigational New Drugs, 13 (1): 89–97, doi:10.1007/BF02614227, PMID 7499115.
- ↑ Hydrazine sulfate:Human/Clinical Studies National Cancer Institute
- 1 2 Chlebowski RT, Bulcavage L, Grosvenor M, et al. (January 1990), "Hydrazine sulfate influence on nutritional status and survival in non-small-cell lung cancer", J. Clin. Oncol., 8 (1): 9–15, PMID 1688616
- 1 2 Piantadosi, S. (1990), "Hazards of small clinical trials" (PDF), Journal of Clinical Oncology, 8 (1): 1, PMID 2295901, retrieved 2009-06-03
- ↑ Loprinzi CL, Goldberg RM, Su JQ, et al. (June 1994), "Placebo-controlled trial of hydrazine sulfate in patients with newly diagnosed non-small-cell lung cancer", J. Clin. Oncol., 12 (6): 1126–9, PMID 8201374
- ↑ Kosty MP, Fleishman SB, Herndon JE, et al. (June 1994), "Cisplatin, vinblastine, and hydrazine sulfate in advanced, non-small-cell lung cancer: a randomized placebo-controlled, double-blind phase III study of the Cancer and Leukemia Group B", J. Clin. Oncol., 12 (6): 1113–20, PMID 8201372
- ↑ Herndon JE, Fleishman S, Kosty MP, Green MR (August 1997), "A longitudinal study of quality of life in advanced non-small cell lung cancer: Cancer and Leukemia Group B (CALGB) 8931", Controlled Clinical Trials, 18 (4): 286–300, doi:10.1016/0197-2456(96)00116-X, PMID 9257067
- ↑ Loprinzi CL, Kuross SA, O'Fallon JR, et al. (June 1994), "Randomized placebo-controlled evaluation of hydrazine sulfate in patients with advanced colorectal cancer", J. Clin. Oncol., 12 (6): 1121–5, PMID 8201373
- ↑ Yavuzsen T, Davis MP, Walsh D, LeGrand S, Lagman R (November 2005), "Systematic review of the treatment of cancer-associated anorexia and weight loss", J. Clin. Oncol., 23 (33): 8500–11, doi:10.1200/JCO.2005.01.8010, PMID 16293879
- ↑ Gagnon B, Bruera E (May 1998), "A review of the drug treatment of cachexia associated with cancer", Drugs, 55 (5): 675–88, doi:10.2165/00003495-199855050-00005, PMID 9585863
- ↑ Hydrazine Hazard Summary, U.S. Environmental Protection Agency, January 2000.
- ↑ Section 9.2.1, Environmental Health Criteria for Hydrazine, International Programme on Chemical Safety, 1987.
- ↑ Hainer, M. I.; et al. (2000), "Fatal hepatorenal failure associated with hydrazine sulfate" (PDF), Annals of Internal Medicine, 133 (11): 877–80, doi:10.7326/0003-4819-133-11-200012050-00011, PMID 11103057.
- ↑ Nagappan, R.; Riddell, T. (2000), "Pyridoxine therapy in a patient with severe hydrazine sulfate toxicity", Critical Care in Medicine, 28 (6): 2116–18, doi:10.1097/00003246-200006000-00076, PMID 10890675.
- ↑ National Cancer Institute (October 1999), "Hydrazine Sulfate", PDQ Complementary/Alternative Medicine
External links
- Proponents
- Critics
- Hydrazine Sulfate: Is It an Anticancer Agent? Quackwatch
- The Penthouse Politics of Cancer: The Promotion of Hydrazine Sulfate and a Medical Conspiracy Theory American Council on Science and Health
- Governmental and medical
- Hydrazine sulfate / Hydrazine sulphate British Columbia Cancer Agency
- What is rocket fuel treatment? Cancer Research UK
- Hydrazine Sulfate American Cancer Society
- Hydrazine Sulfate University of Minnesota, Cancer Information
- Hydrazine Sulfate, Detailed Scientific Review The University of Texas, M. D. Anderson Cancer Center
- Physical and chemical hazards
- Hydrazine sulfate Material safety data sheet
| Salts and esters of the sulfate ion | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2SO4 | He | ||||||||||||||||||
| Li2SO4 | BeSO4 | B | esters ROSO3− (RO)2SO2 |
(NH4)2SO4 N2H6SO4 (NH3OH)2SO4 |
O | F | Ne | ||||||||||||
| Na2SO4 NaHSO4 |
MgSO4 | Al2(SO4)3 Al2SO4(OAc)4 |
Si | P | SO42− | Cl | Ar | ||||||||||||
| K2SO4 KHSO4 |
CaSO4 | Sc2(SO4)3 | Ti(SO4)2 TiOSO4 |
V2(SO4)3 VOSO4 |
CrSO4 Cr2(SO4)3 |
MnSO4 | FeSO4 Fe2(SO4)3 |
CoSO4, Co2(SO4)3 |
NiSO4 | CuSO4 | ZnSO4 | Ga2(SO4)3 | Ge | As | Se | Br | Kr | ||
| RbHSO4 Rb2SO4 |
SrSO4 | Y2(SO4)3 | Zr(SO4)2 | Nb | Mo | Tc | Ru | Rh | PdSO4 | Ag2SO4 | CdSO4 | In2(SO4)3 | SnSO4 | Sb2(SO4)3 | Te | I | Xe | ||
| Cs2SO4 | BaSO4 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2SO4, HgSO4 |
Tl2SO4 | PbSO4 | Bi2(SO4)3 | Po | At | Rn | |||
| Fr | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce2(SO4)3 Ce(SO4)2 |
Pr2(SO4)3 | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb2(SO4)3 | Lu | |||||
| Ac | Th | Pa | U(SO4)2 UO2SO4 |
Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
