Iodine test
| Classification | Colorimetric method |
|---|---|
| Analytes | Starch |
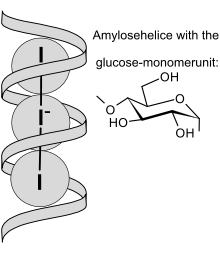
Schematic view of I3− ions embedded in amylose helix
The iodine test is used to test for the presence of starch. When treated with IKI solution—iodine dissolved in an aqueous solution of potassium iodide—the triiodide anion (I3−) complexes with starch, producing an intense blue/purple colour. However, the intensity of the color decreases with increasing temperature and with the presence of water-miscible organic solvents such as ethanol. The test cannot be performed at very low pH due to the hydrolysis of the starch under these conditions.[1]
To put it simply, when the iodine solution comes into contact with starch, it turns dark blue/purple. Otherwise, it will remain brown in color.
See also
References
- ↑ "Iodine Test for Starch". Brilliant Biology Student
Master Biology Labs. Retrieved 2015-12-01.
Further reading
- http://antoine.frostburg.edu/chem/senese/101/redox/faq/starch-as-redox-indicator.shtml
- Vogel's Textbook of Quantitative Chemical Analysis, 5th edition.
External links
- Iodine test at Braukaiser
This article is issued from Wikipedia - version of the 7/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.