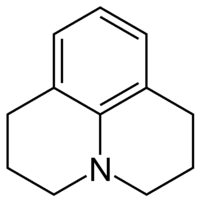
Julolidine
 | |
| Names | |
|---|---|
| IUPAC name
Julolidine | |
| Other names
2,3,6,7-tetrahydro-1H,5H-benzo[ij]quinolizine | |
| Identifiers | |
| 479-59-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 61383 |
| ECHA InfoCard | 100.006.851 |
| PubChem | 68069 |
| |
| |
| Properties | |
| C12H15N | |
| Molar mass | 173.26 g·mol−1 |
| Density | 1.003 g/mL |
| Melting point | 35 °C (95 °F; 308 K) |
| Refractive index (nD) |
1.568 |
| Hazards | |
| Flash point | 110 °C (230 °F; 383 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Julolidine is a heterocyclic aromatic organic compound. It has the formula C12H15N.
Synthesis
The first synthesis of julolidine was first reported by G. Pinkus in 1892.[1]
Applications
This compound and its derivatives have found recent interest as photoconductive materials, chemiluminescence substances, chromogenic substrates in analytical redox reactions, dye intermediates, potential antidepressants and tranquilizers, nonlinear optical materials, high sensitivity photopolymerizable materials, and for improving color stability in photography.
References
- ↑ Pinkus, G. Ueber die Einwirkung von Trimethylenchlorbromid auf einige aromatische Amine und Amide. Berichte der deutschen chemischen Gesellschaft 1892, 25 (2), 2798–2806
External links
- Katritzky, Alan R.; Rachwal, Bogumila; Rachwal, Stanislaw; Abboud, Khalil A. (1996). "Convenient Synthesis of Julolidines Using Benzotriazole Methodology". The Journal of Organic Chemistry. 61 (9): 3117. doi:10.1021/jo9519118. PMID 11667174.
This article is issued from Wikipedia - version of the 1/13/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.