Citrulline
 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-5-(carbamoylamino)pentanoic acid[1] | |
| Identifiers | |
| 627-77-0 13594-51-9 R 372-75-8 S | |
| 3D model (Jmol) | Interactive image Interactive image |
| 3DMet | B01217 |
| 1725417, 1725415 R, 1725416 S | |
| ChEBI | CHEBI:18211 |
| ChEMBL | ChEMBL444814 |
| ChemSpider | 810 553200 R 9367 S |
| DrugBank | DB00155 |
| ECHA InfoCard | 100.006.145 |
| EC Number | 211-012-2 |
| 774677 S | |
| 722 | |
| KEGG | D07706 |
| MeSH | Citrulline |
| PubChem | 833 637599 R 9750 S |
| UNII | 29VT07BGDA |
| |
| |
| Properties | |
| C6H13N3O3 | |
| Molar mass | 175.19 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| log P | −1.373 |
| Acidity (pKa) | 2.508 |
| Basicity (pKb) | 11.489 |
| Thermochemistry | |
| 232.80 J K−1 mol−1 | |
| Std molar entropy (S |
254.4 J K−1 mol−1 |
| Related compounds | |
| Related alkanoic acids |
|
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
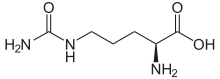
The organic compound citrulline is an α-amino acid. Its name is derived from citrullus, the Latin word for watermelon, from which it was first isolated in 1914 by Koga & Odake. It was finally identified by Wada in 1930.[2] It has the formula H2NC(O)NH(CH2)3CH(NH2)CO2H. It is a key intermediate in the urea cycle, the pathway by which mammals excrete ammonia.
In the body, citrulline is produced as a byproduct of the enzymatic production of nitric oxide from the amino acid arginine, catalyzed by nitric oxide synthase.[3] This is an essential reaction in the body because nitric oxide is an important vasodilator required for regulating blood pressure.
Biosynthesis
Citrulline is made from ornithine and carbamoyl phosphate in one of the central reactions in the urea cycle. It is also produced from arginine as a by-product of the reaction catalyzed by NOS family (NOS; EC 1.14.13.39).[4] It is made from arginine by the enzyme trichohyalin at the inner root sheath and medulla of hair follicles.[5] Arginine is first oxidized into N-hydroxyl-arginine, which is then further oxidized to citrulline concomitant with release of nitric oxide.
Function
Several proteins contain citrulline as a result of a posttranslational modification. These citrulline residues are generated by a family of enzymes called peptidylarginine deiminases (PADs), which convert arginine into citrulline in a process called citrullination or deimination. Proteins that normally contain citrulline residues include myelin basic protein (MBP), filaggrin, and several histone proteins, whereas other proteins, such as fibrin and vimentin are susceptible to citrullination during cell death and tissue inflammation.
Patients with rheumatoid arthritis often have detectable antibodies against proteins containing citrulline. Although the origin of this immune response is not known, detection of antibodies reactive with citrulline (anti-citrullinated protein antibodies) containing proteins or peptides is now becoming an important help in the diagnosis of rheumatoid arthritis.[6]
Circulating citrulline concentration is, in humans, a biomarker of intestinal functionality.[7]
Sources
Citrulline in the form of citrulline malate is sold as a performance-enhancing athletic dietary supplement, which was shown to reduce muscle fatigue in a preliminary human study.[8]
The rind of watermelon (Citrullus lanatus) is a natural source of citrulline, discussed in one report as a precursor to producing nitric oxide which is a physiological factor in relaxing vascular smooth muscle and erectile organs.[9]
See also
References
- ↑ "Citrulline - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification. Retrieved 1 May 2012.
- ↑ Fearon, William Robert (1939). "The Carbamido Diacetyl Reaction: A Test For Citrulline" (PDF). Biochemical Journal. 33 (6): 902–907.
- ↑ "Nos2 - Nitric Oxide Synthase". Uniprot.org. Uniprot Consortium. Retrieved 10 February 2015.
- ↑ Cox M, Lehninger AL, Nelson DR (2000). Lehninger principles of biochemistry (3rd ed.). New York: Worth Publishers. ISBN 1-57259-153-6.
- ↑ Rogers, G. E.; Rothnagel, J. A. (1983). "A sensitive assay for the enzyme activity in hair follicles and epidermis that catalyses the peptidyl-arginine-citrulline post-translational modification". Current Problems in Dermatology. 11: 171–184. PMID 6653155.
- ↑ Coenen D, Verschueren P, Westhovens R, Bossuyt X (March 2007). "Technical and diagnostic performance of 6 assays for the measurement of citrullinated protein/peptide antibodies in the diagnosis of rheumatoid arthritis". Clin. Chem. 53 (3): 498–504. doi:10.1373/clinchem.2006.078063. PMID 17259232.
- ↑ Crenn, P.; et al. (2000). "Post-absorptive plasma citrulline concentration is a marker of intestinal failure in short bowel syndrome patients". Gastroenterology. 119: 1496–505. doi:10.1053/gast.2000.20227.
- ↑ Bendahan D, Mattei JP, Ghattas B, Confort-Gouny S, Le Guern ME, Cozzone PJ (Aug 2002). "Citrulline/malate promotes aerobic energy production in human exercising muscle". Br J Sports Med. 36 (4): 282–9. doi:10.1136/bjsm.36.4.282. PMC 1724533
 . PMID 12145119.
. PMID 12145119. - ↑ Texas A&M University (1 July 2008). "Watermelon may have viagra effect". ScienceDaily. Retrieved 29 November 2014.