Ladderane
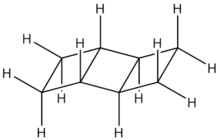
In chemistry, a ladderane is an organic molecule containing two or more fused cyclobutane rings. The name arises from the resemblance of a series of fused cyclobutane rings to a ladder. Numerous synthetic approaches have been developed for the synthesis of ladderane compounds of various lengths.[1] The mechanisms often involve [2 + 2] photocycloadditions, a useful reaction for creating strained 4-membered rings. Naturally occurring ladderanes have been identified as major components of the anammoxosome membrane of the anammox bacteria, Planctomycetes.[2]
Nomenclature
Chain length

Synthetic approaches have yielded ladderanes of varying lengths. A classification system has been developed to describe newly synthesized ladderanes, which is shown at right.[3] The length of the ladderane is described by the number in brackets that precedes the word "ladderane". This is equal to the number of bonds shared by two cyclobutanes (n) plus 1. If the chain becomes long enough, it can cyclize onto itself, forming the band structure of the prismanes and related compounds.
Stereochemistry
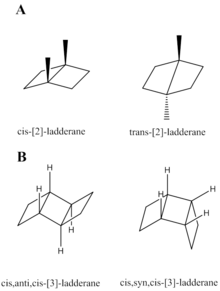
Ladderanes have two forms of stereochemistry.[3] One describes the arrangement of hydrogen atoms between two cyclobutane rings. These hydrogen atoms can be in either the cis- or trans- configuration as shown at right in Figure A. Trans-ladderanes have not been synthesized due to the ring strain in these compounds.
The second form of stereochemistry describes the orientation of three consecutive cyclobutane rings, and therefore is only relevant to ladderanes of n ≥ 2. The two outer rings can be on the same face (syn) or on the opposite face (anti), of the center ring. The syn and anti forms of [3]-ladderane are depicted at right in Figure B.
Synthesis
Various synthetic methods have been used for the laboratory synthesis of ladderane compounds. The three major approaches are (1) dimerization of polyene precursors, (2) the stepwise addition, one or two rings at a time, (3) and oligomerization.[3] Several examples of ladderane synthesis are outlined below.
Dimerization of cyclobutadiene
The dimerization of two cyclobutadienes can generate both the syn and anti ladderane products depending on the reaction conditions.[4] The first step in forming the syn product involves the generation of 1,3-cyclobutadiene by treatment of cis-3,4-dichlorocyclobutene with sodium amalgam. The reactant passes through a metalated intermediate before forming 1,3-cyclobutadiene, which can then dimerize to form the syn-diene. Hydrogenation of the double bonds will form the saturated syn-[3]-ladderane.
To generate the anti product, cis-3,4-dichlorocyclobutene is treated with lithium amalgam.[5] The lithium derivative undergoes a C-C coupling reaction to produce the open dimeric structure. This intermediate reacts to form the anti-diene, which can be hydrogenated to form the final anti-[3]-ladderane product.

Synthesis of n = 3 ladderanes
A different synthetic approach developed by Martin and coworkers has allowed for the synthesis of ladderanes of n = 3.[4] The initial step involves the formation of a [2]-ladderane from the addition of two equivalents of maleic anhydride with acetylene. The remaining two rings are formed from the Ramberg-Backlung ring contraction.

Synthesis of long-chain ladderanes
Ladderanes with lengths up to n = 12 have been synthesized by Mehta and coworkers.[6] This process involves the in situ generation of dicarbomethoxycyclobutadiene from its Fe(CO)3 complex at low temperatures with the addition of ceric(IV) ammonium nitrate (CAN). Generation of the butadiene rapidly forms a mixture of [n]-ladderanes oflengths up to n = 13 with an overall yield of 55%. All of the ladderanes synthesized through this method have one cis,syn,cis structure. This may be a result of the initial dimerization of two cyclobutadienes which preferably forms the syn product, shown below. The further dimerization only produces the anti product due to steric factors.

Dimerization of polyene precursors
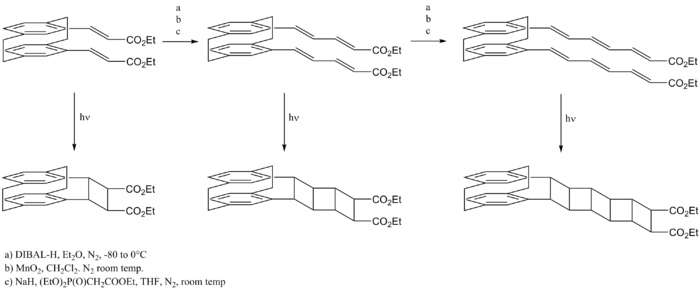
In these reactions, ladderanes are formed from multiple [2 + 2] photocycloaddiitions between the double bonds of two polyenes.[7] A complication that arises from this approach is the reaction of the precursors through alternative, more favorable photoexcitation routes. These side reactions are prevented by the addition of a chemical spacer unit that holds the two polyenes parallel to each other, only allowing [2 + 2] cycloadditions to occur.
A common spacer used in these reactions is the [2.2]paracyclophane system. This is sufficiently rigid and can hold the polyene tails in close enough proximity for the cycloadditions to occur.
Biological background
Ladderanes were first identified in a rare group of anaerobic ammonium oxidizing (anammox) bacteria belonging to the phylum Planctomycetes. These bacteria sequester the catabolic anammox reactions to intracellular compartments called anammoxasomes.[2] The anammox process involves the oxidation of ammonium to nitrogen gas with nitrite as the final electron acceptor. Intermediates in this process are two highly toxic compounds, hydrazine (N2H4) and hydroxylamine (NH2OH). The oxidation process involves the generation of a proton gradient on the intracytoplasmic face of the anammoxasome. Dissipation of the proton gradient is coupled to the phosphorylation of ADP through membrane-bound ATPases.[8]
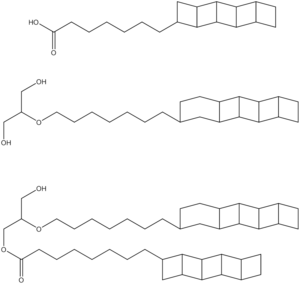
Anammoxasomes are enriched in the ladderane lipids shown at right.[9] Analysis of the anammoxasome membranes from the bacterial species Borcadia anammoxidans and Kuenenia stuttgartiensis has revealed that ladderanes constitute greater than 50% of membrane lipids. The high abundance of ladderane lipids in the anammoxasome results in an exceptionally dense membrane with reduced permeability. The reduced permeability may decrease the passive diffusion of protons across the membrane that would dissipate the electrochemical gradient. This would be especially detrimental to anammox bacteria, due to the relatively slow anammox metabolism. The decreased permeability also sequesters the highly toxic and mutagenic intermediates, hydrazine and hydroxylamine, which can readily diffuse through biomembranes. The loss of these key intermediates would damage key cellular components such as DNA, as well as reduce the catabolic efficiency of the cell.
Synthesis of pentacycloanammoxic acid
A naturally occurring n = 4 ladderane compound, pentacycloanammoxic acid, has been synthesized by Corey and coworkers.[10] The first step in this reaction involves the bromination followed by cyclization of cyclooctatriene to form a cyclohexadiene. This cyclohexadiene is trapped by dibenzyl azodicarboxylate. Functional group modifications are made to produce a cyclobutane which is reacted through a [2 + 2] photocycloaddition with a cyclopentanone to produce a second cyclobutane ring. Protection of the carbonyl group, followed by a N2 extrusion reaction, yields two more fused cyclobutane rings. Further reaction will form a fifth cyclobutane ring with an aliphatic tail, similar to what is found in naturally occurring anammox phospholipids.

References
- ↑ Hopf, Henning; Joel F. Liebman; H. Mark Perks (2009). "Cubanes, fenestranes, ladderanes, prismanes, staffanes and other oligocyclobutanoids". PATAI's Chemistry of Functional Groups. doi:10.1002/9780470682531.pat0337.
- 1 2 Fuerst, John A. (23 May 2005). "Intracelluar Compartmentation in Planctomycetes". Annual Review of Microbiology. 59: 299–328. doi:10.1146/annurev.micro.59.030804.121258. PMID 15910279.
- 1 2 3 Nouri, Dustin H.; Dean J. Tantillo (November 2006). "They Came From the Deep: Syntheses, Applications, and Biology of Ladderanes". Current Organic Chemistry. 10 (16): 2055–2074. doi:10.2174/138527206778742678.
- 1 2 Hopf, Henning (2003). "Step by Step - From Nonnatural to Biological Molecular Ladders". Angewandte Chemie International Edition. 42: 2822–2825. doi:10.1002/anie.200301650.
- ↑ Pettit, R.; J. Henery (1970). "cis-3,4,-Dichlorocyclobutene". Organic Syntheses. 50: 36. doi:10.15227/orgsyn.050.0036.
- ↑ Mehta, Goverdhan; M. Balaji Viswanath; Ajit C. Kunwar (1994). "Characterization of [n]-Ladderanes of Unprecedented Length: A New Record for Fused Carbocyclic Arrays". Journal of Organic Chemistry. 59: 6131–6132. doi:10.1021/jo00100a002.
- ↑ Greiving, Helmut; Henning Hopf; Peter G. Jones; Peter Bubenitschek; Jean Pierre Desvergne; Henri Bouas-Laurent (1994). "Synthesis, photophysical and photochemical properties of four [2.2]'cinnamophane' isomers; highly efficient stereospecific [2 + 2] photocycloaddition". Journal of the Chemical Society, Chemical Communications (9): 1075–1076. doi:10.1039/C39940001075.
- ↑ van Niftrik, Laura A.; John A. Fuerst; Jaap S. Sinninghe Damsté; J. Gijs Kuenen; Mike S.M. Jetten; Marc Strous (2004). "The anammoxosome: an intracytoplasmic compartment in anammox bacteria". Microbiology Letters. 233: 7–13. doi:10.1016/j.femsle.2004.01.044. PMID 15098544.
- ↑ Damsté, Jaap S. Sinnighe; Marc Strous, W. Irene C. Rijpstra, Ellen C. Hopmans, Jan A. J. Geenevasen, Adri C. T. van Duin, Laura A. van Niftrik, Mike S. M. Jetten (17 October 2002). "Linearly concatenated cyclobutane lipids form a dense bacterial membrane". Nature. 419: 708–712. doi:10.1038/nature01128. PMID 12384695. Cite uses deprecated parameter
|coauthors=(help) - ↑ Mascitti, Vincent; E. J. Corey (11 November 1994). "Total Synthesis of (±)-Pentacycloanammoxic Acid". Journal of the American Chemical Society. 126: 15664–15665. doi:10.1021/ja044089a.