Polidocanol
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | topical, subcutaneous injection |
| ATC code | C05BB02 (WHO) |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| Synonyms |
|
| CAS Number |
9002-92-0 3055-99-0 |
| PubChem (CID) | 656641 |
| ChemSpider |
570993 |
| UNII |
0AWH8BFG9A |
| KEGG |
D01993 |
| ChEMBL |
CHEMBL1201751 |
| ECHA InfoCard | 100.105.513 |
| Chemical and physical data | |
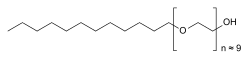
| Formula | C30H62O10 |
| Molar mass | 582.8073 g/mol (predicted) |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Polidocanol is a local anaesthetic and antipruritic component of ointments and bath additives. It relieves itching caused by eczema and dry skin.[1] It is formed by the ethoxylation of dodecanol.
The substance is also used as a sclerosant, an irritant injected to treat varicose veins, under the trade names Asclera, Aethoxysklerol[2] and Varithena.[3] Polidocanol causes fibrosis inside varicose veins, occluding the lumen of the vessel, and reducing the appearance of the varicosity.
The FDA has approved polidocanol injections for the treatment of small varicose (less than 1 mm in diameter) and reticular veins (1 to 3 mm in diameter). Polidocanol works by damaging the cell lining of blood vessels, causing them to close and eventually be replaced by other types of tissue.[4][5]
References
- ↑ "E45 itch relief cream". netdoctor.co.uk. Retrieved 2007-07-12.
- ↑ Sclerotherapy, Laurence Z Rosenberg, MD, eMedicine.com
- ↑ "Varithena™ (polidocanol injectable foam) For Intravenous Use. Full Prescribing Information" (PDF). Biocompatibles, Inc. Retrieved 1 October 2015.
- ↑ Facts and Companies: Varicose Vein Treatment Approved
- ↑ "Asclera Full Prescribing Information in Drug Reference Encyclopedia". Retrieved 2010-04-11.