Lithium iodide
 | |
| Identifiers | |
|---|---|
| 10377-51-2 17023-24-4 (monohydrate) 17023-25-5 (dihydrate) 7790-22-9 (trihydrate) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 59699 |
| ECHA InfoCard | 100.030.735 |
| PubChem | 66321 |
| UNII | S6K2XET783 |
| |
| |
| Properties | |
| LiI | |
| Molar mass | 133.85 g/mol |
| Appearance | White crystalline solid |
| Density | 4.076 g/cm3 (anhydrous) 3.494 g/cm3 (trihydrate) |
| Melting point | 469 °C (876 °F; 742 K) |
| Boiling point | 1,171 °C (2,140 °F; 1,444 K) |
| 1510 g/L (0 °C) 1670 g/L (25 °C) 4330 g/L (100 °C) [1] | |
| Solubility | soluble in ethanol, propanol, ethanediol, ammonia |
| Solubility in methanol | 3430 g/L (20 °C) |
| Solubility in acetone | 426 g/L (18 °C) |
| Refractive index (nD) |
1.955 |
| Thermochemistry | |
| 0.381 J/g K or 54.4 J/mol K | |
| Std molar entropy (S |
75.7 J/mol K |
| Std enthalpy of formation (ΔfH |
-2.02 kJ/g or −270.48 kJ/mol |
| Gibbs free energy (ΔfG˚) |
-266.9 kJ/mol |
| Hazards | |
| Safety data sheet | External MSDS |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions |
Lithium fluoride Lithium chloride Lithium bromide Lithium astatide |
| Other cations |
Sodium iodide Potassium iodide Rubidium iodide Caesium iodide Francium iodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lithium iodide, or LiI, is a compound of lithium and iodine. When exposed to air, it becomes yellow in color, due to the oxidation of iodide to iodine.[2] It crystallizes in the NaCl motif.[3] It can participate in various hydrates.[4]
Applications
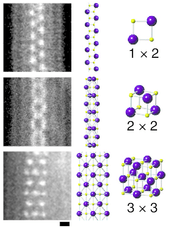
Lithium iodide is used as an electrolyte for high temperature batteries. It is also used for long life batteries as required, for example, by artificial pacemakers. The solid is used as a phosphor for neutron detection.[6] It is also used, in a complex with Iodine, in the electrolyte of dye-sensitized solar cells.
In organic synthesis, LiI is useful for cleaving C-O bonds. For example, it can be used to convert methyl esters to carboxylic acids:[7]
- RCO2CH3 + LiI → RCO2Li + CH3I
Similar reactions apply to epoxides and aziridines.
Lithium iodide was used as a radio contrast agent for X-ray computed tomography imaging studies. Its use was discontinued due to renal toxicity, replaced by organic iodine molecules. Inorganic iodine solutions suffered from hyperosmolarity and high viscosities.[8]
See also
References
- ↑ Patnaik, Pradyot (2002) Handbook of Inorganic Chemicals. McGraw-Hill, ISBN 0-07-049439-8
- ↑ "Lithium iodide" (PDF). ESPI Corp. MSDS.
- ↑ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
- ↑ Wietelmann, Ulrich and Bauer, Richard J. (2005) "Lithium and Lithium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH: Weinheim. doi:10.1002/14356007.a15_393.
- ↑ Senga, Ryosuke; Suenaga, Kazu (2015). "Single-atom electron energy loss spectroscopy of light elements". Nature Communications. 6: 7943. doi:10.1038/ncomms8943. PMC 4532884
 . PMID 26228378.
. PMID 26228378. - ↑ Nicholson, K. P.; et al. (1955). "Some lithium iodide phosphors for slow neutron detection". Br. J. Appl. Phys. 6 (3): 104–106. doi:10.1088/0508-3443/6/3/311.
- ↑ Charette, André B.; Barbay, J. Kent and He, Wei (2005) "Lithium Iodide" in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons. doi:10.1002/047084289X.rl121.pub2
- ↑ Lusic, Hrvoje; Grinstaff, Mark W. (2013). "X-ray-Computed Tomography Contrast Agents". Chemical Reviews. 113 (3): 1641. doi:10.1021/cr200358s. PMID 23210836.
External links
| Wikimedia Commons has media related to Lithium iodide. |
- "Webelements – Lithium Iodide". Retrieved 2005-09-16. -->
- "Composition of LITHIUM IODIDE – NIST". Retrieved 2006-02-03. -->