McLafferty rearrangement
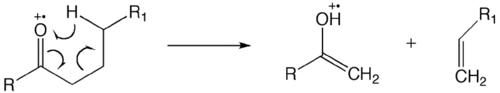
The McLafferty rearrangement is a reaction observed in mass spectrometry. It is sometimes found that a molecule containing a keto-group undergoes β-cleavage, with the gain of the γ-hydrogen atom. This rearrangement may take place by a radical or ionic mechanism.
The reaction
A description of the reaction was first published by the American chemist Fred McLafferty in 1959.[1][2][3]
See also
References
- ↑ F. W. McLafferty (1959). "Mass Spectrometric Analysis. Molecular Rearrangements". Anal. Chem. 31 (1): 82–87. doi:10.1021/ac60145a015.
- ↑ Gross ML (2004). "Focus in honor of Fred McLafferty, 2003 Distinguished Contribution awardee, for the discovery of the "McLafferty Rearrangement"". J. Am. Soc. Mass Spectrom. 15 (7): 951–5. doi:10.1016/j.jasms.2004.05.009. PMID 15234352.
- ↑ Nibbering NM (2004). "The McLafferty rearrangement: a personal recollection". J. Am. Soc. Mass Spectrom. 15 (7): 956–8. doi:10.1016/j.jasms.2004.04.025. PMID 15234353.
Further reading
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "McLafferty rearrangement".
External links
This article is issued from Wikipedia - version of the 11/4/2014. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.