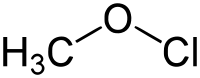
Methyl hypochlorite
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(Chlorooxy)methane | |||
| Systematic IUPAC name
Methyl hypochlorite | |||
| Other names
Hypochlorous acid methyl ester; Methoxy chloride | |||
| Identifiers | |||
| 593-78-2 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 71388 | ||
| PubChem | 79056 | ||
| |||
| |||
| Properties | |||
| CH3ClO | |||
| Molar mass | 66.48 g·mol−1 | ||
| Appearance | Gas | ||
| Odor | Pungent | ||
| Density | 1.058 g/cm3 | ||
| Melting point | −120.4 °C (−184.7 °F; 152.8 K) | ||
| Boiling point | 9.18 °C (48.52 °F; 282.33 K) | ||
| Decomposes | |||
| Refractive index (nD) |
1.343 | ||
| Hazards | |||
| R-phrases | R3 R8 R23/24/25 R35 | ||
| NFPA 704 | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Methyl hypochlorite is an unstable and highly toxic compound that can be produced from reacting methanol with hypochlorous acid. It is extremely unstable and can decompose explosively giving off toxic fumes. Its instability is caused by the oxidizing power of the hypochlorite group, which can easily undergo a redox reaction with the methyl group.
This article is issued from Wikipedia - version of the 11/2/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.


