Methylenomycin A
 | |
 | |
| Names | |
|---|---|
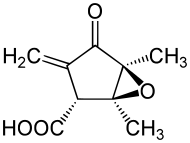
| IUPAC name
(1S,2R,5S)-1,5-dimethyl-3-methylidene-4-oxo-6-oxabicyclo[3.1.0]hexane-2-carboxylic acid | |
| Identifiers | |
| 52775-76-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 9074545 |
| PubChem | 10899285 |
| |
| |
| Properties | |
| C9H10O4 | |
| Molar mass | 182.19 g mol−1 |
| Boiling point | 341.2 °C (646.2 °F; 614.3 K) |
| Hazards | |
| Flash point | 141.2 °C (286.2 °F; 414.3 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Methylenomycin A is a cyclopentanoid antibiotic produced by Streptomyces coelicolor A3(2) that is effective against both Gram-negative and Gram-positive bacteria.[1][2] Methylenomycins are naturally produced in two variants: A and B.
See also
References
- ↑ Brian P, Riggle PJ, Santos RA, Champness WC (June 1996). "Global negative regulation of Streptomyces coelicolor antibiotic synthesis mediated by an absA-encoded putative signal transduction system". J. Bacteriol. 178 (11): 3221–31. PMC 178074
 . PMID 8655502.
. PMID 8655502. - ↑ Hobbs G, Obanye AI, Petty J, et al. (March 1992). "An integrated approach to studying regulation of production of the antibiotic methylenomycin by Streptomyces coelicolor A3(2)". J. Bacteriol. 174 (5): 1487–94. PMC 206543
 . PMID 1537793.
. PMID 1537793.
This article is issued from Wikipedia - version of the 6/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.