Muconic acid
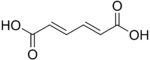 | |
| Names | |
|---|---|
| IUPAC name
(2E,4E)-Hexa-2,4-dienedioic acid | |
| Other names
(E,E)-Muconic acid | |
| Identifiers | |
| 3588-17-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27036 |
| ChemSpider | 4512358 |
| ECHA InfoCard | 100.020.659 |
| EC Number | 222-724-8 |
| PubChem | 5356793 |
| |
| |
| Properties | |
| C6H6O4 | |
| Molar mass | 142.11 g·mol−1 |
| Appearance | Crystalline prisms |
| Density | 1.366 g/mL |
| Melting point | 194 to 195 °C (381 to 383 °F; 467 to 468 K) (cis,cis-form, prisms from ethanol), 301 °C (trans,trans-form, prisms from water), 190–191 °C (cis,trans-form, needles from hot water)[3] |
| Boiling point | 345 °C (653 °F; 618 K) |
| 1 g/L | |
| Hazards | |
| Main hazards | Irritant |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Muconic acid is a dicarboxylic acid. There are three isomeric forms designated trans,trans-muconic acid, cis,trans-muconic acid, and cis,cis-muconic acid which differ by the geometry around the double bonds.


-Muconic-acid-3D-balls.png)
-Muconic-acid-3D-balls.png)
-Muconic-acid-3D-balls.png)
trans,trans cis,trans cis,cis
trans,trans-Muconic acid is a metabolite of benzene in humans. The determination of its concentration in urine is therefore used as a biomarker of occupational or environmental exposure to benzene.[4][5] Synthetically, trans,trans-muconic acid can be prepared from adipic acid.[6]
cis,cis-Muconic acid is produced by some bacteria by the enzymatic degradation of various aromatic chemical compounds.
The bioproduction of muconic acid is of interest because of its potential use as a platform chemical for the production of several valuable consumer bioplastics including nylon-6,6, polyurethane, and polyethylene terephthalate (PET).[7]
See also
Notes
- ↑ Merck Index, 11th Edition, 6210
- ↑ trans,trans-Muconic acid at Sigma-Aldrich
- ↑ Merck Index, 12th Edition (1996), 6381, p.1079
- ↑ Wiwanitkit V, Soogarun S, Suwansaksri J (2007). "A correlative study on red blood cell parameters and urine trans, trans-muconic acid in subjects with occupational benzene exposure". Toxicologic pathology. 35 (2): 268–9. doi:10.1080/01926230601156278. PMID 17366320.
- ↑ Weaver VM, Davoli CT, Heller PJ, et al. (1996). "Benzene exposure, assessed by urinary trans,trans-muconic acid, in urban children with elevated blood lead levels". Environ. Health Perspect. Brogan &. 104 (3): 318–23. doi:10.2307/3432891. JSTOR 3432891. PMC 1469300
 . PMID 8919771.
. PMID 8919771. - ↑ Organic Syntheses, Coll. Vol. 3, p.623 (1955); Vol. 26, p.57 (1946). Online copy
- ↑ Curran KA, Leavitt JM, Karim AS, Alper HS. "Metabolic engineering of muconic acid production in Saccharomyces cerevisiae.". Metab. Eng. doi:10.1016/j.ymben.2012.10.003. PMID 23164574.