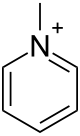
Methylpyridinium
 | |
| Names | |
|---|---|
| IUPAC name
1-Methylpyridinium | |
| Other names
N-Methylpyridinium | |
| Identifiers | |
| 694-56-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 13008 |
| PubChem | 13597 |
| |
| |
| Properties | |
| C6H8N+ | |
| Molar mass | 94.134 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methylpyridinium is a chemical compound which is the quaternary ammonium compound derived from the N-methylation of pyridine. It is found in some coffee products.[1] It is not present in unroasted coffee beans, but is formed during roasting from its precursor chemical, trigonelline.[1] It is under investigation by scientists regarding its potential anti-carcinogenic properties,[2] particularly an effect on colon cancer.[1]
Ionic liquid
The chloride of N-methylpyridinium behaves as an ionic liquid in the molten state. Its properties with different mixtures of zinc chloride have been characterised by several authors in the temperature range 150 – 200 °C (423 – 473 K).[3], [4], [5], [6]
See also
- Pyridinium
- 4-Caffeoyl-1,5-quinide
-
 Coffee portal
Coffee portal
References
- 1 2 3 "Highly Active Compound Found In Coffee May Prevent Colon Cancer". ScienceDaily. Oct 15, 2003. Retrieved 2012. Check date values in:
|access-date=(help) - ↑ Boettler, U; Volz, N; Pahlke, G; Teller, N; Kotyczka, C; Somoza, V; Stiebitz, H; Bytof, G; et al. (2011). "Coffees rich in chlorogenic acid or N-methylpyridinium induce chemopreventive phase II-enzymes via the Nrf2/ARE pathway in vitro and in vivo". Molecular nutrition & food research. 55 (5): 798–802. doi:10.1002/mnfr.201100115. PMID 21448860.
- ↑ Simonis, L.; Coppe, C.; Glibert, J.; Claes, P. (1986). "Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – I. Phase diagram and heats of mixing". Thermochimica acta. 99: 223–232. doi:10.1016/0040-6031(86)85285-6.
- ↑ Claes, P.; Simonis, L.; Glibert, J. (1986). "Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – II. Specific mass, electrical conductivity and viscosity". Electrochimica acta. 31 (12): 1525–1530. doi:10.1016/0013-4686(86)87071-2.
- ↑ Claes, P. F.; Coppe, C. R.; Simonis, L. A.; Glibert, J. E. (1987). "Properties of mixtures of zinc chloride and N-methylpyridinium chloride in the molten state – III. Solubility of hydrogen chloride under atmospheric pressure and comparison with zinc chloride - N-ethylpyridinium bromide mixtures". Journal of Chemical and Engineering Data. 32 (1): 70–72. doi:10.1021/je00047a020.
- ↑ Marković, R.; Minić, D. M. (1997). "Conductometric and thermal studies of fused Zn (II) salts containing methyl substituted pyridinium cations". Materials chemistry and physics. 50 (1): 20–24. doi:10.1016/S0254-0584(97)80178-2.
This article is issued from Wikipedia - version of the 6/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
