Nitarsone
 | |
| Names | |
|---|---|
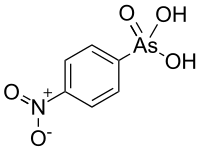
| IUPAC name
(4-Nitrophenyl)arsonic acid | |
| Other names
(p-Nitrophenyl)arsonic acid; 4-Nitrobenzenearsonic acid | |
| Identifiers | |
| 98-72-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 60190 |
| ECHA InfoCard | 100.002.451 |
| PubChem | 66826 |
| |
| |
| Properties | |
| C6H6AsNO5 | |
| Molar mass | 247.04 g·mol−1 |
| Melting point | 298–300 °C (568–572 °F; 571–573 K) (decomposes) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nitarsone is an organoarsenic compound that is used in poultry production as a feed additive to increase weight gain, improve feed efficiency, and prevent blackhead disease.[1] It is marketed as Histostat by Zoetis, a publicly traded subsidiary of Pfizer.[2]
Nitarsone is one of four arsenical animal drugs approved by the U.S. Food and Drug Administration for use in poultry, along with roxarsone, arsanilic acid, and carbarsone.[3] Following the 2011 suspension of roxarsone sales in the United States, however, it is believed to be the only arsenical animal drug currently marketed in the U.S.[3][4] In September 2013, the FDA announced that Zoetis and Fleming Laboratories would voluntarily withdraw current roxarsone, arsanilic acid, and carbarsone approvals, leaving only nitarsone approvals in place.[5] When the withdrawals occurred, nitarsone was only arsenical approved for use in food animals in the U.S. In 2015 FDA withdrew the approval of using nitarsone in animal feeds. The ban came into effect at the end of 2015.[6]
References
- ↑ U.S. Food and Drug Administration. "Animal Drugs @ FDA".
- ↑ Zoetis. "Histostat: Type A Medicated Feed Article".
- 1 2 U.S. Food and Drug Administration (June 8, 2011). "Questions and Answers Regarding 3-Nitro (Roxarsone)".
- ↑ Sabrina Tavernise (May 11, 2013). "Study Finds an Increase in Arsenic Levels in Chicken". New York Times.
- ↑ U.S. Food and Drug Administration (September 20, 2011). "FDA Response to Citizen Petition on Arsenic-based Animal Drugs".
- ↑ U.S. Food and Drug Administration (April 1, 2015). "FDA Announces Pending Withdrawal of Approval of Nitarsone".