Norbornene
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bicyclo[2.2.1]hept-2-ene | |||
| Other names
Norbornylene Norcamphene | |||
| Identifiers | |||
| 498-66-8 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 9925 | ||
| ECHA InfoCard | 100.007.152 | ||
| EC Number | 207-866-0 | ||
| PubChem | 10352 | ||
| |||
| |||
| Properties | |||
| C7H10 | |||
| Molar mass | 94.16 g·mol−1 | ||
| Appearance | White solid | ||
| Melting point | 42 to 46 °C (108 to 115 °F; 315 to 319 K) | ||
| Boiling point | 96 °C (205 °F; 369 K) | ||
| Hazards | |||
| NFPA 704 | |||
| Flash point | −15 °C (5 °F; 258 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
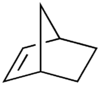
Norbornene or norbornylene or norcamphene is a bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between C-3 and C-6. The molecule carries a double bond which induces significant ring strain and significant reactivity.
Norbornene, like many of its derivatives, is made by a Diels-Alder reaction of cyclopentadiene and ethylene.[2][3] Related bicyclics are norbornadiene which has the same carbon skeleton but with two double bonds and norbornane which is completely saturated without double bonds.
Norbornene undergoes an acid-catalyzed hydration reaction with water to form norborneol. This reaction is of great interest to chemists studying non-classical ions.
Uses
Norbornene does not have as many practical uses as ethylene or other commodity chemicals. It is utilized to make pharmaceutical intermediates, pesticide compounds, specialty fragrances and in general organic synthesis. When combined with ethylene, norbornene will react and turn into a cyclic olefin copolymer.
Norbornene is commonly used in transition metal catalysis to affect migration of electrophilic transition metals [4] It is also commonly used in transition metal catalysis as a sacrificial hydrogen acceptor. In the Catellani reaction it is used as an ortho-directing mediator.
Polynorbornenes
Norbornenes are important monomers in ring-opening metathesis polymerizations (ROMP) with for instance the Grubbs' catalyst. Polynorbornenes are polymers with high glass transition temperatures and high optical clarity.[5]
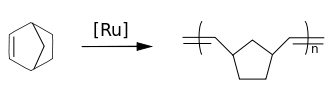
In addition to ROMP polymerization, norbornene monomers also undergo vinyl-addition polymerization.
Polynorbornene is used mainly in the rubber industry for anti-vibration (rail, building, industry), anti-impact (personal protective equipment, shoe parts, bumpers) and grip improvement (toy tires, racing tires, transmission systems, transports systems for copiers, feeders, etc.)
- Reachable performances: Loss factors (tan delta) larger than 3, rebounds of less than 1%, tear strengths of 50 N/mm2, friction coefficients of 2 and more, Shore hardness between 4 and 90 Shore A.
- Second main application: oil-binding system with absorption capability of hydrocarbons, 10 times of own weight
Ethylidene norbornene is a related monomer derived from cyclopentadiene and butadiene.
References
- ↑ Norbornene MSDS
- ↑ Paul Binger, Petra Wedemann, and Udo H. Brinker. "Cyclopropene: A New Simple Synthesis and its Diels-Alder Reaction with Cyclopentadiene". Org. Synth.; Coll. Vol., 10, p. 231
- ↑ Masaji Oda, Takeshi Kawase, Tomoaki Okada, and Tetsuya Enomoto. "2-Cyclohexene-1,4-dione". Org. Synth.; Coll. Vol., 9, p. 186
- ↑ Thansandote, Praew; Chong, Eugene; Feldmann, Kai-Oliver; Lautens, Mark (21 May 2010). "Palladium-Catalyzed Domino C−C/C−N Coupling Using a Norbornene Template: Synthesis of Substituted Benzomorpholines, Phenoxazines, and Dihydrodibenzoxazepines". The Journal of Organic Chemistry. 75 (10): 3495–3498. doi:10.1021/jo100408p. PMID 20423091.
- ↑ Mol, J. C. Journal of Molecular Catalysis A: Chemical 2004, 213, 39.